|
Sulfurous Acid
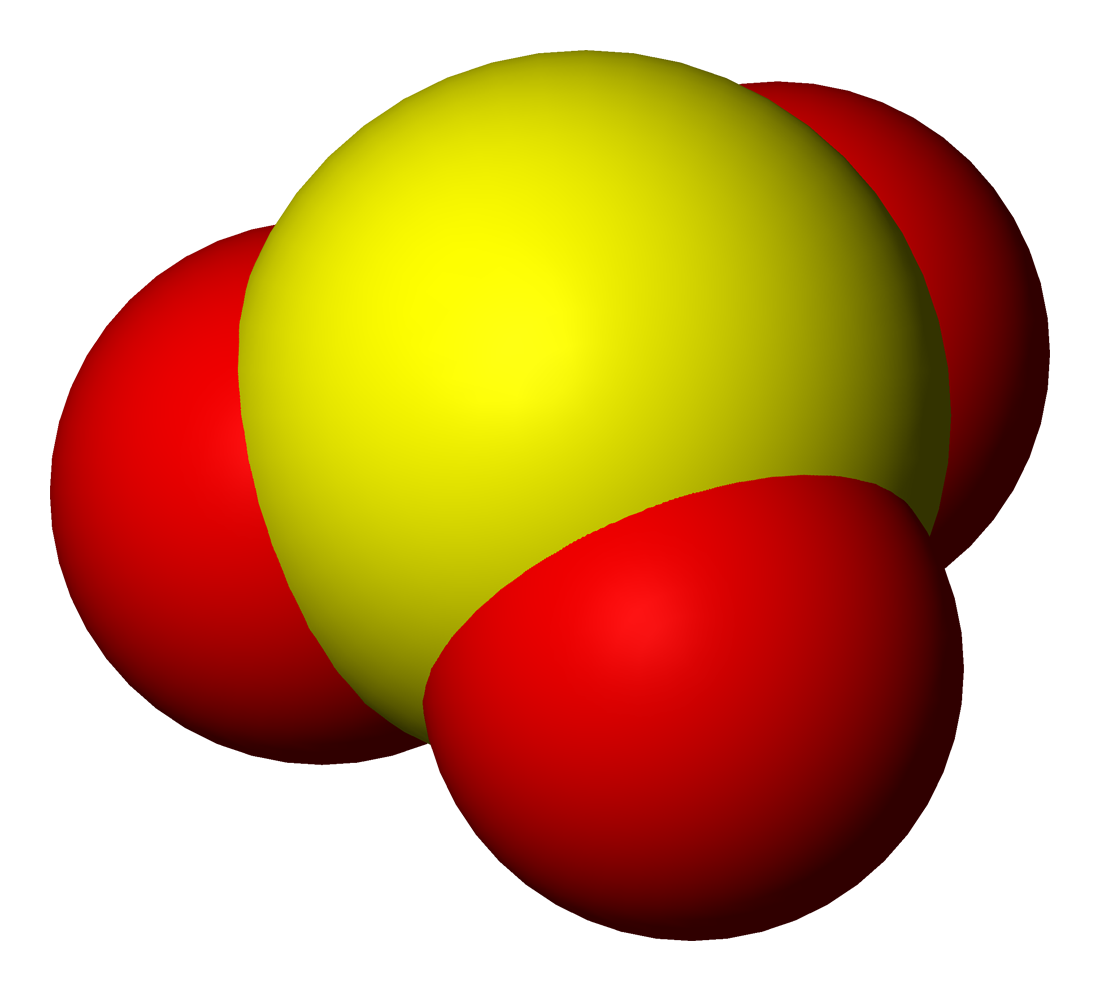
Sulfurous acid (also sulfuric(IV) acid, sulphurous acid (UK), sulphuric(IV) acid (UK)) is the chemical compound with the formula . There is no evidence that sulfurous acid exists in solution, but the molecule has been detected in the gas phase. The conjugate bases of this elusive acid are, however, common anions, bisulfite (or hydrogen sulfite) and sulfite. Sulfurous acid is an intermediate species in the formation of acid rain from sulfur dioxide. Raman spectra of solutions of sulfur dioxide in water show only signals due to the molecule and the bisulfite ion, . The intensities of the signals are consistent with the following equilibrium: 17O NMR spectroscopy provided evidence that solutions of sulfurous acid and protonated sulfites contain a mixture of isomers, which is in equilibrium: Attempts to concentrate the solutions of sulfurous acid simply reverses the equilibrium, producing sulfur dioxde and water vapor. A clathrate with the formul a has been crystallised. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bisulfite
The bisulfite ion (IUPAC-recommended nomenclature: hydrogensulfite) is the ion . Salts containing the ion are also known as "sulfite lyes". Sodium bisulfite is used interchangeably with sodium metabisulfite (Na2S2O5). Sodium metabisulfite dissolves in water to give a solution of Na+. :Na2S2O5 + H2O → 2Na SO3 Structure The bisulfite anion exists in solution as a mixture of two tautomers. One tautomer has the proton attached to one of the three oxygen centers. In the second tautomer the proton resides on sulfur. The S-protonated tautomer has ''C''3v symmetry. The O-protonated tautomer has only Cs symmetry. Reactions Tautomerization There exist two tautomers of bisulfite. They interconvert readily but can be characterized individually by various spectroscopic methods. They have been observed by 17O NMR spectroscopy: :HSO3− SO2(OH)− K = 4.2 Acid-base reactions Solutions of bisulfite are typically prepared by treatment of sulfur dioxide with aqueous base: :SO ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clathrate
A clathrate is a chemical substance consisting of a lattice that traps or contains molecules. The word ''clathrate'' is derived from the Latin (), meaning ‘with bars, latticed’. Most clathrate compounds are polymeric and completely envelop the guest molecule, but in modern usage clathrates also include host–guest complexes and inclusion compounds.Atwood, J. L. (2012) "Inclusion Compounds" in ''Ullmann's Encyclopedia of Industrial Chemistry''. Wiley-VCH, Weinheim. According to IUPAC, clathrates are inclusion compounds "in which the guest molecule is in a cage formed by the host molecule or by a lattice of host molecules." The term refers to many molecular hosts, including calixarenes and cyclodextrins and even some inorganic polymers such as zeolites. Clathrates can be divided into two categories: clathrate hydrates and inorganic clathrates. Each clathrate is made up of a framework and guests that reside the framework. Most common clathrate crystal structures can be compos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Compounds
Hydrogen compounds are compounds containg the element hydrogen. In these compounds, hydrogen can form in the +1 and -1 oxidation states. Hydrogen can form compounds both ionically and in covalent substances. It is a part of many organic compounds such as hydrocarbons as well as water and other organic substances. The ion is often called a proton because it has one proton and no electrons, although the proton does not move freely. Brønsted–Lowry acids are capable of donating ions to bases. Covalent and organic compounds While is not very reactive under standard conditions, it does form compounds with most elements. Hydrogen can form compounds with elements that are more electronegative, such as halogens (F, Cl, Br, I), or oxygen; in these compounds hydrogen takes on a partial positive charge. When bonded to a more electronegative element, particularly fluorine, oxygen, or nitrogen, hydrogen can participate in a form of medium-strength noncovalent bonding with another elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid ( sulfurous acid) is elusive, its salts are widely used. Sulfites are substances that naturally occur in some foods and the human body. They are also used as regulated food additives. When in food or drink, sulfites are often lumped together with sulfur dioxide.SeREGULATION (EU) No 1169/2011 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL/ref> Structure The structure of the sulfite anion can be described with three equivalent resonance structures. In each resonance structure, the sulfur atom is double-bonded to one oxygen atom with a formal charge of zero (neutral), and sulfur is singly bonded to the other two oxygen atoms, which each carry a formal charge of −1, together accounting for the −2 charge on the anion. There is also a non-bonded lone pair on the sulfur, so the structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
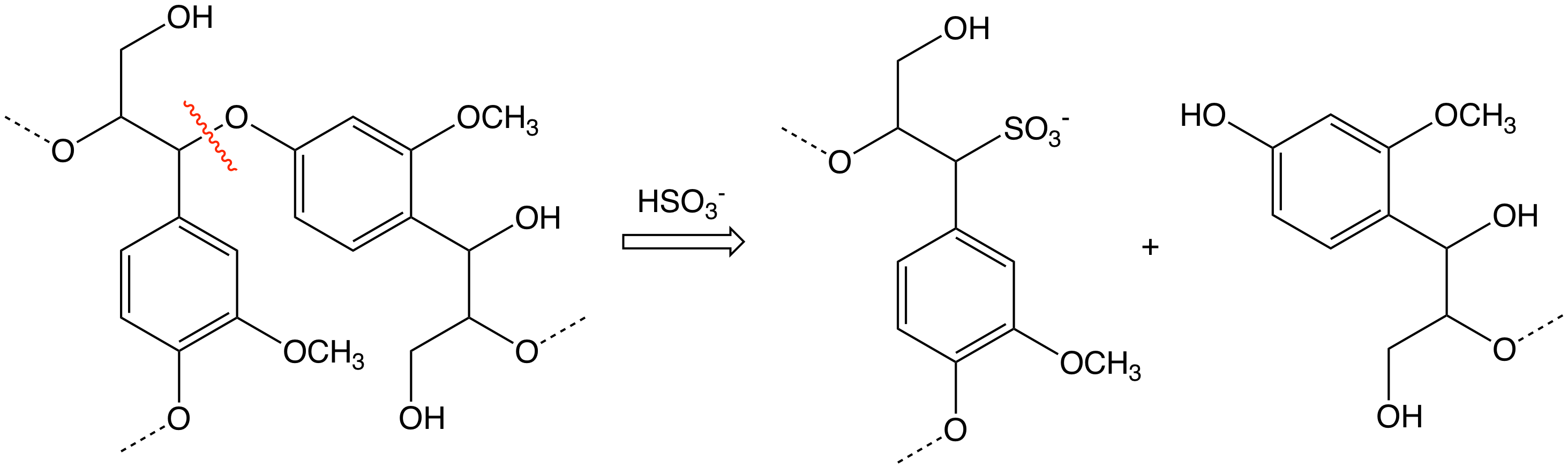
Sulfite Process
The sulfite process produces wood pulp that is almost pure cellulose fibers by treating wood chips with solutions of sulfite and bisulfite ions. These chemicals cleave the bonds between the cellulose and lignin components of the lignocellulose. A variety of sulfite/bisulfite salts are used, including sodium (Na+), calcium (Ca2+), potassium (K+), magnesium (Mg2+), and ammonium (NH4+). The lignin is converted to lignosulfonates, which are soluble and can be separated from the cellulose fibers. For the production of cellulose, the sulfite process competes with the Kraft process which produces stronger fibers and is less environmentally costly. History The use of wood to make pulp for paper began with the development of mechanical pulping in the 1840s by Charles Fenerty in Nova ScotiaBurger, Peter 'Charles Fenerty and his Paper Invention''. Toronto: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pulp (paper)
Pulp is a lignocellulosic fibrous material prepared by chemically or mechanically separating cellulose fibers from wood, fiber crops, waste paper, or rags. Mixed with water and other chemical or plant-based additives, pulp is the major raw material used in papermaking and the industrial production of other paper products. History Before the widely acknowledged invention of papermaking by Cai Lun in China around 105 AD, paper-like writing materials such as papyrus and amate were produced by ancient civilizations using plant materials which were largely unprocessed. Strips of bark or bast material were woven together, beaten into rough sheets, dried, and polished by hand. Pulp used in modern and traditional papermaking is distinguished by the process which produces a finer, more regular slurry of cellulose fibers which are pulled out of solution by a screen and dried to form sheets or rolls. The earliest paper produced in China consisted of bast fibers from the paper mulberr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid. Spelling "Sulfate" is the spelling recommended by IUPAC, but "sulphate" was traditionally used in British English. Structure The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry is the same as that of methane. The sulfur atom is in the +6 oxidation state while the four oxygen atoms are each in the −2 state. The sulfate ion carries an overall charge of −2 and it is the conjugate base of the bisulfate (or hydrogensulfate) ion, , which is in turn the conjugate base of , sulfuric acid. Organic sulfate esters, such as dimethyl sulfate, are covalent compounds and esters of sulfuric acid. The tetrahedral molecular geometry of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula . It is a colorless, odorless and viscous liquid that is miscible with water. Pure sulfuric acid does not exist naturally on Earth due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air. Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid, but to the contrary dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus the reverse procedure of adding water to the acid should not be performed since the heat released may boi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidisation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogenation, C=C (and other) bonds ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reducing Agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ). Examples of substances that are commonly reducing agents include the Earth metals, formic acid, oxalic acid, and sulfite compounds. In their pre-reaction states, reducers have extra electrons (that is, they are by themselves reduced) and oxidizers lack electrons (that is, they are by themselves oxidized). This is commonly expressed in terms of their oxidation states. An agent's oxidation state describes its degree of loss of electrons, where the higher the oxidation state then the fewer electrons it has. So initially, prior to the reaction, a reducing agent is typically in one of its lower possible oxidation states; its oxidation state increases during the reaction while that of the oxidizer decreases. Thus in a redox reaction, the agent whose oxidation state increases, that "loses/Electron donor, donates electrons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |