|
Sulfoquinovose
Sulfoquinovose, also known as 6-sulfoquinovose and 6-deoxy-6-sulfo-D-glucopyranose, is a monosaccharide sugar that is found as a building block in the sulfolipid sulfoquinovosyl diacylglycerol (SQDG). Sulfoquinovose is a sulfonic acid derivative of glucose, the sulfonic acid group is introduced into the sugar by the enzyme UDP-sulfoquinovose synthase UDP-sulfoquinovose synthase () is an enzyme that catalyzes the chemical reaction :UDP-glucose + sulfite \rightleftharpoons UDP-6-sulfoquinovose + H2O Thus, the two substrates of this enzyme are UDP-glucose and sulfite, whereas its two products ... (SQD1). Sulfoquinovose is degraded through a metabolic process termed sulfoglycolysis. The half-life for mutarotation of sulfoquinovose at pD 7.5 and 26C is 299 minutes. See also * Sulfolipid * Sulfoglycolysis References {{Reflist Sulfonic acids Deoxy sugars Monosaccharides Sulfate esters ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfoglycolysis
Sulfoglycolysis is a catabolic process in primary metabolism in which sulfoquinovose (6-deoxy-6-sulfonato-glucose) is metabolized to produce energy and carbon-building blocks. Sulfoglycolysis pathways occur in a wide variety of organisms, and enable key steps in the degradation of sulfoquinovosyl diacylglycerol (SQDG), a sulfolipid found in plants and cyanobacteria into sulfite and sulfate. Sulfoglycolysis converts sulfoquinovose (C6H12O8S−) into various smaller metabolizable carbon fragments such as pyruvate and dihydroxyacetone phosphate that enter central metabolism. The free energy is used to form the high-energy molecules ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide). Unlike glycolysis, which allows metabolism of all carbons in glucose, some sulfoglycolysis pathways convert only a fraction of the carbon content of sulfoquinovose into smaller metabolizable fragments; the remaineder is excreted as C3-sulfonates 2,3-dihydroxypropanesulfonate ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfoglycolysis
Sulfoglycolysis is a catabolic process in primary metabolism in which sulfoquinovose (6-deoxy-6-sulfonato-glucose) is metabolized to produce energy and carbon-building blocks. Sulfoglycolysis pathways occur in a wide variety of organisms, and enable key steps in the degradation of sulfoquinovosyl diacylglycerol (SQDG), a sulfolipid found in plants and cyanobacteria into sulfite and sulfate. Sulfoglycolysis converts sulfoquinovose (C6H12O8S−) into various smaller metabolizable carbon fragments such as pyruvate and dihydroxyacetone phosphate that enter central metabolism. The free energy is used to form the high-energy molecules ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide). Unlike glycolysis, which allows metabolism of all carbons in glucose, some sulfoglycolysis pathways convert only a fraction of the carbon content of sulfoquinovose into smaller metabolizable fragments; the remaineder is excreted as C3-sulfonates 2,3-dihydroxypropanesulfonate ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
UDP-sulfoquinovose Synthase
UDP-sulfoquinovose synthase () is an enzyme that catalyzes the chemical reaction :UDP-glucose + sulfite \rightleftharpoons UDP-6-sulfoquinovose + H2O Thus, the two substrates of this enzyme are UDP-glucose and sulfite, whereas its two products are UDP-6- sulfoquinovose and H2O. In a subsequent reaction catalyzed by sulfoquinovosyl diacylglycerol synthase, the sulfoquinovose portion of UDP-sulfoquinovose is combined with diacyglycerol to produce the sulfolipid sulfoquinovosyl diacylglycerol (SQDG). This enzyme belongs to the family of hydrolases, specifically those acting on carbon-sulfur bonds. The systematic name of this enzyme class is UDP-6-sulfo-6-deoxyglucose sulfohydrolase. Other names in common use include sulfite:UDP-glucose sulfotransferase, and UDP-sulfoquinovose synthase. This enzyme participates in nucleotide sugars metabolism and glycerolipid metabolism. The 3-dimensional structure of the enzyme is known from Protein Data Bank The Protein Data Bank (PDB) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfoquinovosyl Diacylglycerol
Sulfoquinovosyl diacylglycerols, abbreviated SQDG, are a class of sulfur-containing but phosphorus-free lipids ( sulfolipids) found in many photosynthetic organisms. Discovery, structure and chemical properties In 1959 A. A. Benson and coworkers discovered a new sulfur-containing lipid in plants and identified it as sulfoquinovosyl diacylglycerol (SQDG). The sulfolipid structure was defined as 1,2-di-''O''-acyl-3-O-(6-deoxy-6-sulfo-α-D-glucopyranosyl)-''sn''-glycerol (SQDG). The distinctive feature of this substance is carbon bonded directly to sulfur as C-SO3. Sulfonic acids of this type are chemically stable and strong acids. Biological occurrence and functions SQDGs have been found in all photosynthetic plants, algae, cyanobacteria, purple sulfur and non-sulfur bacteria and is localised in the thylakoid membranes, being the most saturated glycolipid. SQDGs have been found to be closely associated with certain membrane proteins. In some cases the (electrostatic) interacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
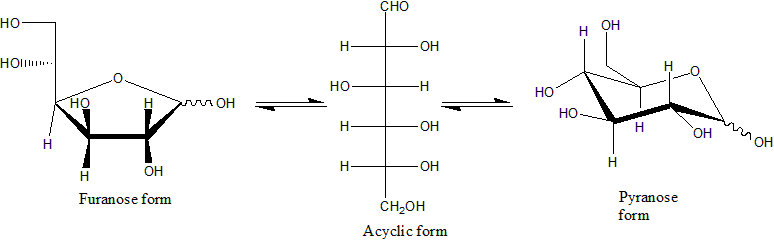
Monosaccharide
Monosaccharides (from Greek ''monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built. They are usually colorless, water-soluble, and crystalline solids. Contrary to their name (sugars), only some monosaccharides have a sweet taste. Most monosaccharides have the formula (though not all molecules with this formula are monosaccharides). Examples of monosaccharides include glucose (dextrose), fructose (levulose), and galactose. Monosaccharides are the building blocks of disaccharides (such as sucrose and lactose) and polysaccharides (such as cellulose and starch). The table sugar used in everyday vernacular is itself a disaccharide sucrose comprising one molecule of each of the two monosaccharides D-glucose and D-fructose. Each carbon atom that supports a hydroxyl group is chiral, except those at the end of the chain. This gives rise to a number of isomeric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfolipid
Sulfolipids are a class of lipids which possess a sulfur-containing functional group. An abundant sulfolipid is sulfoquinovosyl diacylglycerol, which is composed of a glycoside of sulfoquinovose and diacylglycerol. In plants, sulfoquinovosyl diacylglycerides (SQDG) are important members of the sulfur cycle. Other important sulfolipids include sulfatide and seminolipid, each of which are sulfated glycolipids. Sulfolipids have been implicated in the functions of two of the core components of the photosynthetic electron transport chain and while not necessarily essential, might have a protective function when the photosynthetic apparatus is under stress. Must see * Sulfatide * Galactolipid Galactolipids are a type of glycolipid whose sugar group is galactose. They differ from glycosphingolipids in that they do not have nitrogen in their composition. They are the main part of plant membrane lipids where they substitute phospholipids ... *Phospholipid *Glycolipid References L ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfolipid
Sulfolipids are a class of lipids which possess a sulfur-containing functional group. An abundant sulfolipid is sulfoquinovosyl diacylglycerol, which is composed of a glycoside of sulfoquinovose and diacylglycerol. In plants, sulfoquinovosyl diacylglycerides (SQDG) are important members of the sulfur cycle. Other important sulfolipids include sulfatide and seminolipid, each of which are sulfated glycolipids. Sulfolipids have been implicated in the functions of two of the core components of the photosynthetic electron transport chain and while not necessarily essential, might have a protective function when the photosynthetic apparatus is under stress. Must see * Sulfatide * Galactolipid Galactolipids are a type of glycolipid whose sugar group is galactose. They differ from glycosphingolipids in that they do not have nitrogen in their composition. They are the main part of plant membrane lipids where they substitute phospholipids ... *Phospholipid *Glycolipid References L ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monosaccharides
Monosaccharides (from Greek ''monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built. They are usually colorless, water-soluble, and crystalline solids. Contrary to their name (sugars), only some monosaccharides have a sweet taste. Most monosaccharides have the formula (though not all molecules with this formula are monosaccharides). Examples of monosaccharides include glucose (dextrose), fructose (levulose), and galactose. Monosaccharides are the building blocks of disaccharides (such as sucrose and lactose) and polysaccharides (such as cellulose and starch). The table sugar used in everyday vernacular is itself a disaccharide sucrose comprising one molecule of each of the two monosaccharides D-glucose and D-fructose. Each carbon atom that supports a hydroxyl group is chiral, except those at the end of the chain. This gives rise to a number of isomer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double sugars, are molecules made of two bonded monosaccharides; common examples are sucrose (glucose + fructose), lactose (glucose + galactose), and maltose (two molecules of glucose). White sugar is a refined form of sucrose. In the body, compound sugars are hydrolysed into simple sugars. Longer chains of monosaccharides (>2) are not regarded as sugars, and are called oligosaccharides or polysaccharides. Starch is a glucose polymer found in plants, the most abundant source of energy in human food. Some other chemical substances, such as glycerol and sugar alcohols, may have a sweet taste, but are not classified as sugar. Sugars are found in the tissues of most plants. Honey and fruits are abundant natural sources of simple sugars. Suc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonic Acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, , a tautomer of sulfurous acid, . Salt (chemistry), Salts or esters of sulfonic acids are called sulfonates. Preparation Aryl sulfonic acids are produced by the process of sulfonation. Usually the sulfonating agent is sulfur trioxide. A large scale application of this method is the production of alkylbenzenesulfonic acids: :RC6H5 + SO3 -> RC6H4SO3H In this reaction, sulfur trioxide is an electrophile and the arene is the nucleophile. The reaction is an example of electrophilic aromatic substitution. Alkyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Derivative (chemistry)
In chemistry, a derivative is a compound that is derived from a similar compound by a chemical reaction. In the past, derivative also meant a compound that ''can be imagined to'' arise from another compound, if one atom or group of atoms is replaced with another atom or group of atoms, but modern chemical language now uses the term structural analog for this meaning, thus eliminating ambiguity. The term "structural analogue" is common in organic chemistry. In biochemistry, the word is used for compounds that at least theoretically can be formed from the precursor compound. Chemical derivatives may be used to facilitate analysis. For example, melting point (MP) analysis can assist in identification of many organic compounds. A crystalline derivative may be prepared, such as a semicarbazone or 2,4-dinitrophenylhydrazone (derived from aldehydes or ketones), as a simple way of verifying the identity of the original compound, assuming that a table of derivative MP values is available ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose in cell walls, the most abundant carbohydrate in the world. In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as starch and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form of glucose is -glucose, while -glucose is produced synthetically in comparatively small amounts and is less biologically active. Glucose is a monosaccharide containing six carbon atoms and an aldehyde group, and is therefore an aldohexose. The glucose molecule can exist in an open-chain (acyclic) as well as ring (cyclic) form. Gluco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |