|
Strontium Bromide
Strontium bromide is a chemical compound with a formula SrBr2. At room temperature it is a white, odourless, crystalline powder. Strontium bromide imparts a bright red colour in a flame test, showing the presence of strontium ions. It is used in flares and also has some pharmaceutical uses. Preparation SrBr2 can be prepared from strontium hydroxide and hydrobromic acid. :\mathrm Alternatively strontium carbonate can also be used as strontium source. :\mathrm These reactions give hexahydrate of SrBr2, which decomposes to dihydrate at 89 °C. At 180 °C anhydrous SrBr2 is obtained.Dale L. Perry, Sidney L. Phillips: ''Handbook of Inorganic Compounds''. CRC Press, 1995, , (). Structure At room temperature, strontium bromide adopts a crystal structure with a tetragonal unit cell and space group ''P''4/''n''. This structure is referred to as α-SrBr2 and is isostructural with EuBr2 and USe2. Around 920 K (650 °C), α-SrBr2 undergoes a first-order solid-solid phase tr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strontium
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is exposed to air. Strontium has physical and chemical properties similar to those of its two vertical neighbors in the periodic table, calcium and barium. It occurs naturally mainly in the minerals celestine and strontianite, and is mostly mined from these. Both strontium and strontianite are named after Strontian, a village in Scotland near which the mineral was discovered in 1790 by Adair Crawford and William Cruickshank; it was identified as a new element the next year from its crimson-red flame test color. Strontium was first isolated as a metal in 1808 by Humphry Davy using the then newly discovered process of electrolysis. During the 19th century, strontium was mostly used in the production of sugar from sugar beets (see strontian p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrobromic Acid
Hydrobromic acid is a strong acid formed by dissolving the diatomic molecule hydrogen bromide (HBr) in water. "Constant boiling" hydrobromic acid is an aqueous solution that distills at and contains 47.6% HBr by mass, which is 8.77 mol/L. Hydrobromic acid has a p''K''a of −9, making it a stronger acid than hydrochloric acid, but not as strong as hydroiodic acid. Hydrobromic acid is one of the strongest mineral acids known. Uses Hydrobromic acid is mainly used for the production of inorganic bromides, especially the bromides of zinc, calcium, and sodium. It is a useful reagent for generating organobromine compounds. Certain ethers are cleaved with HBr. It also catalyzes alkylation reactions and the extraction of certain ores. Industrially significant organic compounds prepared from hydrobromic acid include allyl bromide, tetrabromobis(phenol), and bromoacetic acid. HBr almost uniquely participates in anti-Markovnikov hydrohalogenation of alkenes. The resulting 1-bro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Transition
In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of matter: solid, liquid, and gas, and in rare cases, plasma. A phase of a thermodynamic system and the states of matter have uniform physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point. Types of phase transition At the phase transition point for a substance, for instance the boiling point, the two phases involved - liquid and vapor, have identic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Transition
In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of matter: solid, liquid, and gas, and in rare cases, plasma. A phase of a thermodynamic system and the states of matter have uniform physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point. Types of phase transition At the phase transition point for a substance, for instance the boiling point, the two phases involved - liquid and vapor, have identic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium Diselenide
Uranium diselenide is a compound of uranium and selenium. It has a β form that has orthorhombic crystal system. The family of crystals it matches is PbCl2. The dimensions of the unit cell are a: 7.455 Å, b: 4.2320 Å, c= 8.964 Å. The compound has the unusual property of ferromagnetism, but only if the temperature is below 14 K. Tellurium can be substituted for selenium in varying quantities, expanding the lattice and increasing the ferromagnetic Curie temperature In physics and materials science, the Curie temperature (''T''C), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, which can (in most cases) be replaced by induced magnetism. The Cur .... References {{Selenides Uranium(IV) compounds Selenides Dichalcogenides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Europium(II) Bromide
Europium(II) bromide is a crystalline compound of one europium atom and two bromine atoms. Europium(II) bromide is a white powder at room temperature, and odorless. Europium dibromide is hygroscopic Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance .... Reactions Europium(II) bromide is known to be involved in three reactions: :2 EuBr3 + Eu → 3 EuBr2 (requires a temperature of 800-900 °C) :2 EuBr3 → 2 EuBr2 + Br2 (requires a temperature of 900-1000 °C) :Eu + HgBr2 → EuBr2 + Hg (requires a temperature of 700-800 °C) References {{Lanthanide halides Bromides Europium(II) compounds Lanthanide halides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isostructural
Isostructural chemical compounds have similar chemical structures. "Isomorphous" when used in the relation to crystal structures is not synonymous: in addition to the same atomic connectivity that characterises isostructural compounds, isomorphous substances crystallise in the same space group and have the same unit cell dimensions. The IUCR definitionIUCR Online Dictionary of CRYSTALLOGRAPHY, "Isostructural crystals"/ref> used by crystallographers is: Examples include: *I- Gold(I) bromide is isostructural with gold(I) chloride *Borazine is isostructural with benzene * Indium(I) bromide is isostructural with β-thallium(I) iodide and has a distorted rock salt structure. Many minerals are isostructural when they differ only in the nature of a cation. Compounds which are isoelectronic usually have similar chemical structures. For example, methane, CH4, and the ammonium ion, NH4+, are isoelectric and are isostructural as both have a tetrahedral structure. The C-H and N-H bond length ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Space Group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchanged. In three dimensions, space groups are classified into 219 distinct types, or 230 types if chiral copies are considered distinct. Space groups are discrete cocompact groups of isometries of an oriented Euclidean space in any number of dimensions. In dimensions other than 3, they are sometimes called Bieberbach groups. In crystallography, space groups are also called the crystallographic or Fedorov groups, and represent a description of the symmetry of the crystal. A definitive source regarding 3-dimensional space groups is the ''International Tables for Crystallography'' . History Space groups in 2 dimensions are the 17 wallpaper groups which have been known for several centuries, though the proof that the list was complete was only ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
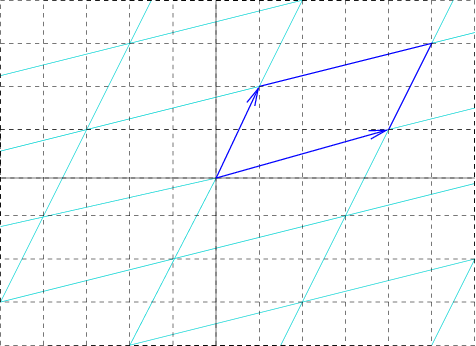
Unit Cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessarily have unit size, or even a particular size at all. Rather, the primitive cell is the closest analogy to a unit vector, since it has a determined size for a given lattice and is the basic building block from which larger cells are constructed. The concept is used particularly in describing crystal structure in two and three dimensions, though it makes sense in all dimensions. A lattice can be characterized by the geometry of its unit cell, which is a section of the tiling (a parallelogram or parallelepiped) that generates the whole tiling using only translations. There are two special cases of the unit cell: the primitive cell and the conventional cell. The primitive cell is a unit cell corresponding to a single lattice point, it is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetragonal Crystal System
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square base (''a'' by ''a'') and height (''c'', which is different from ''a''). Bravais lattices There are two tetragonal Bravais lattices: the primitive tetragonal and the body-centered tetragonal. The base-centered tetragonal lattice is equivalent to the primitive tetragonal lattice with a smaller unit cell, while the face-centered tetragonal lattice is equivalent to the body-centered tetragonal lattice with a smaller unit cell. Crystal classes The point groups that fall under this crystal system are listed below, followed by their representations in international notation, Schoenflies notation, orbifold notation, Coxeter notation and mineral examples.Hurlbut, Cornelius S.; Klein, Cornelis, 1985, ''Manual of Mineralogy'', 20th ed., pp. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystal, crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns that repeat along the principal directions of Three-dimensional space (mathematics), three-dimensional space in matter. The smallest group of particles in the material that constitutes this repeating pattern is the unit cell of the structure. The unit cell completely reflects the symmetry and structure of the entire crystal, which is built up by repetitive Translation (geometry), translation of the unit cell along its principal axes. The translation vectors define the nodes of the Bravais lattice. The lengths of the principal axes, or edges, of the unit cell and the angles between them are the lattice constants, also called ''lattice parameters'' or ''cell parameters''. The symmetry properties of the crystal are described by the con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achieve perfect dryness; anhydrous compounds gradually absorb water from the atmosphere so they must be stored carefully. Solids Many salts and solids can be dried using heat, or under vacuum. Desiccators can also be used to store reagents in dry conditions. Common desiccants include phosphorus pentoxide and silica gel. Chemists may also require dry glassware for sensitive reactions. This can be achieved by drying glassware in an oven, by flame, or under vacuum. Dry solids can be produced by freeze-drying, which is also known as lyophilization. Liquids or solvents In many cases, the presence of water can prevent a reaction from happening, or cause undesirable products to form. To prevent this, anhydrous solvents must be used when performi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |