|
Stachybotrys Oenanthes
''Stachybotrys'' () is a genus of molds, hyphomycetes or asexually reproducing, filamentous fungi, now placed in the family Stachybotryaceae. The genus was erected by August Carl Joseph Corda in 1837. Historically, it was considered closely related to the genus ''Memnoniella'', because the spores are produced in slimy heads rather than in dry chains. Recently, the synonymy of the two genera is generally accepted. Most ''Stachybotrys'' species inhabit materials rich in cellulose. The genus has a widespread distribution and contains about 50 species. The name comes from the Greek words σταχυς ''stakhus'' (ear of grain, stalk, stick; metaphorically, progeny) and βότρυς ''botrus'' (cluster or bunch as in grapes, trusses). The most infamous species, '' S. chartarum'' (previously known as ''S. atra'') and ''S. chlorohalonata'', are known as black mold or toxic black mold in the U.S., and are frequently associated with poor indoor air quality that arises after fungal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
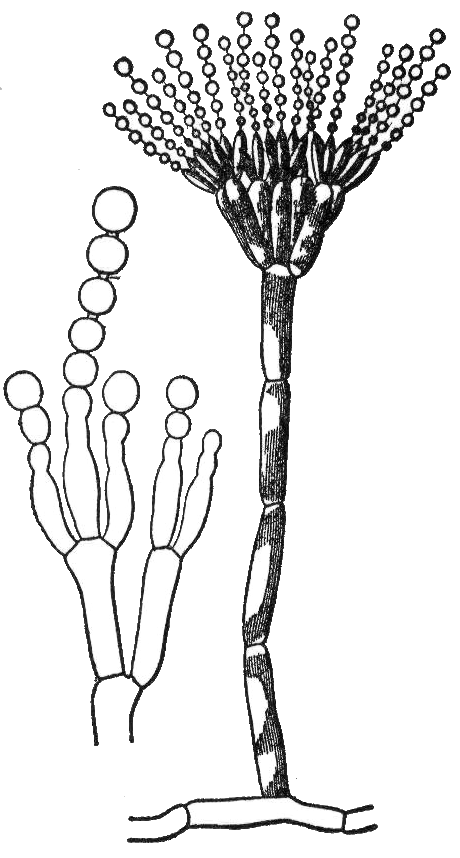
Conidiophore
A conidium ( ; ), sometimes termed an asexual chlamydospore or chlamydoconidium (), is an Asexual reproduction, asexual, non-motility, motile spore of a fungus. The word ''conidium'' comes from the Ancient Greek word for dust, ('). They are also called mitospores due to the way they are generated through the cellular process of mitosis. The two new haploid cells are genetically identical to the haploid parent, and can develop into new organisms if conditions are favorable, and serve in biological dispersal. Asexual reproduction in ascomycetes (the phylum Ascomycota) is by the formation of conidia, which are borne on specialized stalks called conidiophores. The Morphology (biology), morphology of these specialized conidiophores is often distinctive between species and, before the development of molecular techniques at the end of the 20th century, was widely used for identification of (''e.g.'' ''Metarhizium#Species, Metarhizium'') species. The terms microconidia and macroconidi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stachybotrys Chartarum
''Stachybotrys chartarum'' (, ), also known as black mold or toxic black mold, is a species of microfungus that produces its conidia in slime heads. It is sometimes found in soil and grain, but the mold is most often detected in cellulose-rich building materials, such as gypsum-based drywall and wallpaper, from damp or water-damaged buildings. Taxonomy The fungus was originally described scientifically in 1818 by Christian Gottfried Ehrenberg as a member of the genus '' Stilbospora''. His diagnosis emphasized the form of the spores, which he described as minute, sub-opaque, ovate, and agglomerated into subconcentric, water-soluble irregular clusters. He noted that the fungus adheres to paper, sometimes forming circles dotted with black. Stanley Hughes transferred the taxon to '' Stachybotrys'' in 1958. This genus was circumscribed in 1832 by Czech mycologist August Carl Joseph Corda, with ''Stachybotrys atra'' assigned as its type species. The species concept of ''Stachybotrys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
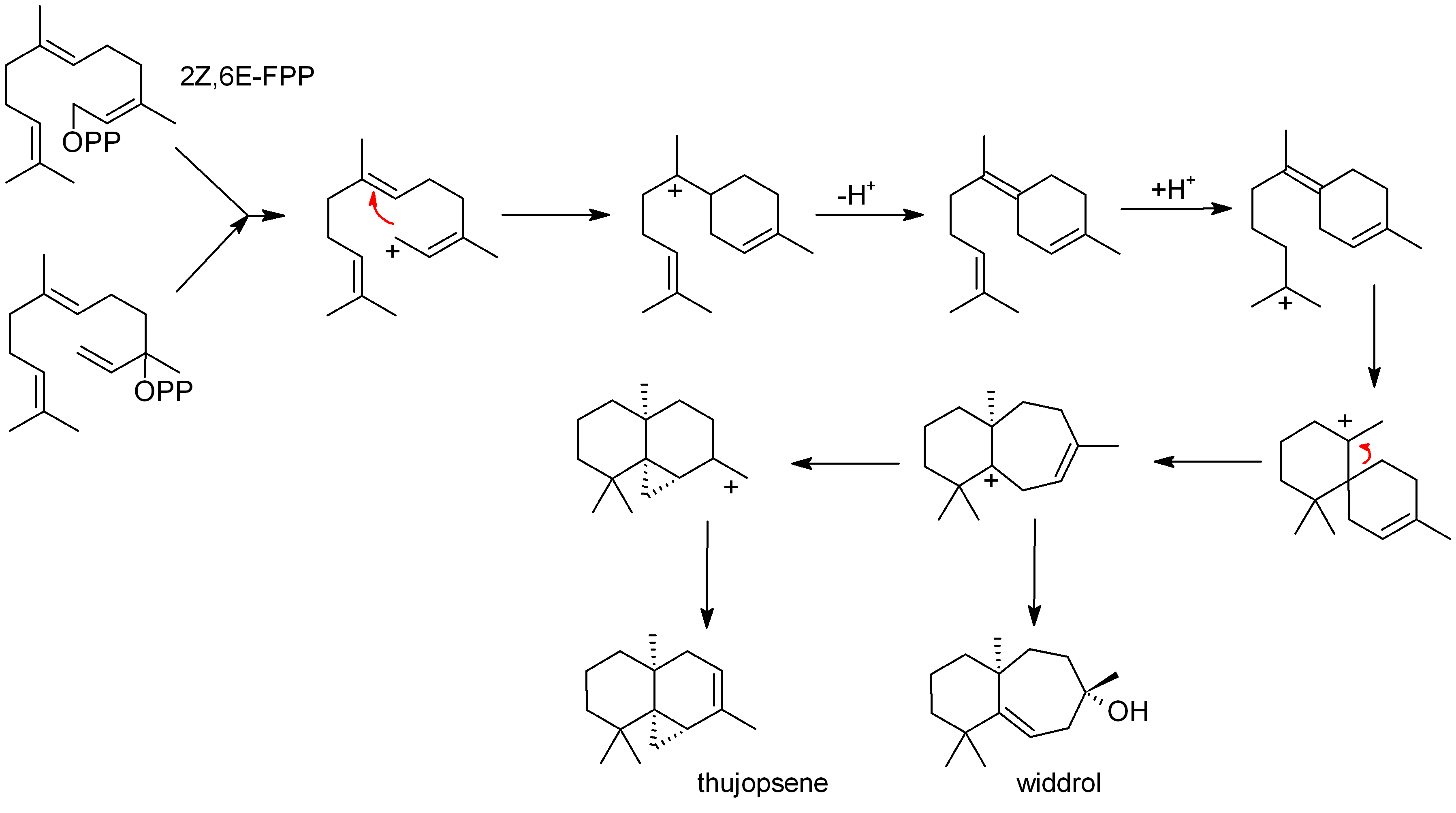
Thujopsene
Thujopsene is a natural chemical compound, classified as a sesquiterpene, with the molecular formula C15H24. Thujopsene is found in the essential oil of a variety of conifers, in particular ''Juniperus cedrus'' and ''Thujopsis dolabrata'' in which it comprises around 2.2% of the weight of the heartwood. Biosynthesis Thujopsene is biosynthesized from farnesyl pyrophosphate Farnesyl pyrophosphate (FPP), also known as farnesyl diphosphate (FDP), is an intermediate in the biosynthesis of terpenes and terpenoids such as sterols and carotenoids. It is also used in the synthesis of CoQ (part of the electron transport cha ... (FPP): References {{reflist Hydrocarbons Sesquiterpenes Cyclopropanes Tricyclic compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-methyl-2-butanol
3-Methyl-2-butanol (IUPAC name, commonly called ''sec''-isoamyl alcohol) is an organic chemical compound. It is used as a solvent and an intermediate in the manufacture of other chemicals. The compound is one of the eight isomers of pentyl alcohol An amyl alcohol is any of eight alcohols with the formula C5H12O. A mixture of amyl alcohols (also called amyl alcohol) can be obtained from fusel alcohol. Amyl alcohol is used as a solvent and in esterification, by which is produced amyl acetate .... References Alcohol solvents Alkanols Secondary alcohols {{Alcohol-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-methyl-1-butanol
Isoamyl alcohol is a colorless liquid with the formula , specifically (H3C–)2CH–CH2–CH2–OH. It is one of several isomers of amyl alcohol (pentanol). It is also known as isopentyl alcohol, isopentanol, or (in the IUPAC recommended nomenclature) 3-methyl-butan-1-ol. An obsolete name for it was isobutyl carbinol. Isoamyl alcohol is an ingredient in the production of banana oil, an ester found in nature and also produced as a flavouring in industry. It is a common fusel alcohol, produced as a major by-product of ethanol fermentation. Occurrence Isoamyl alcohol is one of the components of the aroma of ''Tuber melanosporum'', the black truffle. The compound has also been identified as a chemical in the pheromone used by hornets to attract other members of the hive to attack. Isoamyl acetate is a component of the natural aroma of bananas, especially the Gros Michel variety. Extraction from fusel oil Isoamyl alcohol can be separated from fusel oil by either of two methods: sh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-butanol
1-Butanol, also known as butan-1-ol or ''n''-butanol, is a primary alcohol with the chemical formula C4H9OH and a linear structure. Isomers of 1-butanol are isobutanol, butan-2-ol and ''tert''-butanol. The unmodified term butanol usually refers to the straight chain isomer. 1-Butanol occurs naturally as a minor product of the ethanol fermentation of sugars and other saccharides and is present in many foods and drinks... It is also a permitted artificial flavorant in the United States, used in butter, cream, fruit, rum, whiskey, ice cream and ices, candy, baked goods, and cordials. It is also used in a wide range of consumer products. The largest use of 1-butanol is as an industrial intermediate, particularly for the manufacture of butyl acetate (itself an artificial flavorant and industrial solvent). It is a petrochemical derived from propylene. Estimated production figures for 1997 are: United States 784,000 tonnes; Western Europe 575,000 tonnes; Japan 225,000 ton ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volatile Organic Compound
Volatile organic compounds (VOCs) are organic compounds that have a high vapour pressure at room temperature. High vapor pressure correlates with a low boiling point, which relates to the number of the sample's molecules in the surrounding air, a trait known as volatility. VOCs are responsible for the odor of scents and perfumes as well as pollutants. VOCs play an important role in communication between animals and plants, e.g. attractants for pollinators, protection from predation, and even inter-plant interactions. Some VOCs are dangerous to human health or cause harm to the environment. Anthropogenic VOCs are regulated by law, especially indoors, where concentrations are the highest. Most VOCs are not acutely toxic, but may have long-term chronic health effects. Definitions Diverse definitions of the term VOC are in use. Canada Health Canada classifies VOCs as organic compounds that have boiling points roughly in the range of . The emphasis is placed on commonly encountere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |