|
Stabilizers For Polymers
Polymer stabilizers (British: polymer stabilisers) are chemical additives which may be added to polymeric materials, such as plastics and rubbers, to inhibit or retard their degradation. Common polymer degradation processes include oxidation, UV-damage, thermal degradation, ozonolysis, combinations thereof such as photo-oxidation, as well as reactions with catalyst residues, dyes, or impurities. All of these degrade the polymer at a chemical level, via chain scission, uncontrolled recombination and cross-linking, which adversely affects many key properties such as strength, malleability, appearance and colour. Stabilizers are used at all stages of the polymer life-cycle. They allow plastic items to be produced faster and with fewer defects, extend their useful lifespan, and facilitate their recycling. However they also continue to stabilise waste plastic, causing it to remain in the environment for longer. Many different types of plastic exist and each may be vulnerable to seve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer (; Greek ''poly-'', "many" + '' -mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'', mean ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass Transition Temperature
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased. ISO 11357-2: Plastics – Differential scanning calorimetry – Part 2: Determination of glass transition temperature (1999). An amorphous solid that exhibits a glass transition is called a glass. The reverse transition, achieved by supercooling a viscous liquid into the glass state, is called vitrification. The glass-transition temperature ''T''g of a material characterizes the range of temperatures over which this glass transition occurs (as an experimental definition, typically marked as 100 s of relaxation time). It is always lower than the melting temperature, ''T''m, of the crystalline state of the material, if one exists. Hard plastics like polystyrene and poly(methyl methacrylate) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butylated Hydroxytoluene
Butylated hydroxytoluene (BHT), also known as dibutylhydroxytoluene, is a lipophilic organic compound, chemically a derivative of phenol, that is useful for its antioxidant properties. BHT is widely used to prevent free radical-mediated oxidation in fluids (e.g. fuels, oils) and other materials, and the regulations overseen by the U.S. F.D.A.—which considers BHT to be " generally recognized as safe"—allow small amounts to be added to foods. Despite this, and the earlier determination by the National Cancer Institute that BHT was noncarcinogenic in an animal model, societal concerns over its broad use have been expressed. BHT has also been postulated as an antiviral drug, but as of December 2022 , use of BHT as a drug is not supported by the scientific literature and it has not been approved by any drug regulatory agency for use as an antiviral. Natural occurrence Phytoplankton, including the green algae '' Botryococcus braunii'', as well as three different cyanobact ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (— O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule. Phenols are both synthesized industrially and produced by plants and microorganisms. Properties Acidity Phenols are more acidic than typical alcohols. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12). Deprotonation of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides (aryloxides according to the IUPAC Gold Book). Condensation with aldehydes and ketones Phenols are susceptible to Electrophilic aromatic substitutions. Condensation with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroperoxide
Hydroperoxides or peroxols are compounds containing the hydroperoxide functional group (ROOH). If the R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. Organic hydroperoxides can either intentionally or unintentionally initiate explosive polymerisation in materials with unsaturated chemical bonds. Properties The O−O bond length in peroxides is about 1.45 Å, and the R−O−O angles (R = H, C) are about 110° (water-like). Characteristically, the C−O−O−H dihedral angles are about 120°. The O−O bond is relatively weak, with a bond dissociation energy of , less than half the strengths of C−C, C−H, and C−O bonds. Hydroperoxides are typically more volatile than the corresponding alcohols: * ''tert''-BuOOH (b.p. 36°C) vs ''tert''-BuOH (b.p. 82-83°C) * CH3OOH (b.p. 46°C) vs CH3OH (b.p. 65°C * cumene hydroperoxide (b.p. 153°C) vs cumyl alcohol (b.p. 202°C) Misc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rate Determining Step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the corresponding rate equation (for comparison with the experimental rate law) is often simplified by using this approximation of the rate-determining step. In principle, the time evolution of the reactant and product concentrations can be determined from the set of simultaneous rate equations for the individual steps of the mechanism, one for each step. However, the analytical solution of these differential equations is not always easy, and in some cases numerical integration may even be required. The hypothesis of a single rate-determining step can greatly simplify the mathematics. In the simplest case the initial step is the slowest, and the overall rate is just the rate of the first step. Also, the rate equations for mechanisms with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chain Propagation
Chain propagation (sometimes referred to as propagation) is a process in which a reactive intermediate is continuously regenerated during the course of a chemical chain reaction. For example, in the chlorination of methane, there is a two-step propagation cycle involving as chain carriers a chlorine atom and a methyl radical IUPAC Gold Book which are regenerated alternately: :•Cl + CH4 → HCl + •CH3 :•CH3 + Cl2 → CH3Cl + •Cl The two steps add to give the equation for the overall chain reaction: :CH4 + Cl2 → CH3Cl + HCl. Polymerization In a |
Hydrogen Atom Abstraction
In chemistry, a hydrogen atom abstraction or hydrogen atom transfer (HAT) is any chemical reaction in which a hydrogen free radical (neutral hydrogen atom) is abstracted from a substrate according to the general equation: :X^\bullet + H-Y -> X-H + Y^\bullet Examples of HAT reactions are oxidative reactions in general, hydrocarbon combustion, and reactions involving cytochrome P450 containing an iron(V)-oxo unit. The abstractor (X•) is usually a radical species itself, but it may also be a closed-shell In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom ... species such as chromyl chloride. HAT can take place through proton-coupled electron transfer. A synthetic example is found in iron zeolites, which stabilize alpha-oxygen. References {{Reflist Chemical reactions Reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homolysis (chemistry)
In chemistry, homolysis () or homolytic fission is the dissociation of a molecular bond by a process where each of the fragments (an atom or molecule) retains one of the originally bonded electrons. During homolytic fission of a neutral molecule with an even number of electrons, two free radicals will be generated. That is, the two electrons involved in the original bond are distributed between the two fragment species. Bond cleavage is also possible by a process called heterolysis. The energy involved in this process is called bond dissociation energy (BDE). BDE is defined as the " enthalpy (per mole) required to break a given bond of some specific molecular entity by homolysis," symbolized as ''D''. BDE is dependent on the strength of the bond, which is determined by factors relating to the stability of the resulting radical species. Because of the relatively high energy required to break bonds in this manner, homolysis occurs primarily under certain circumstances: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
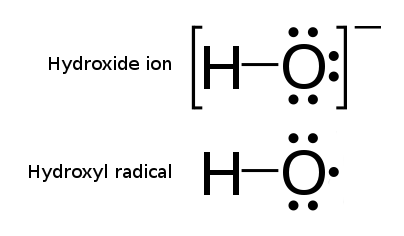
Hydroxyl Radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also important in the field of radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and SCC in coolant systems subjected to radioactive environments. In organic synthesis, hydroxyl radicals are most commonly generated by photolysis of 1-hydroxy-2(1''H'')-pyridinethione. Notation The unpaired electron of the hydroxyl radical is officially represented by a middle dot, •, beside the O. Biology Hydroxyl radicals can occasionally be produced as a byproduct of immune action. Macrophages and microglia most frequently generate this compound wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical Scavenger
A scavenger in chemistry is a chemical substance added to a mixture in order to remove or de-activate impurities and unwanted reaction products, for example oxygen, to make sure that they will not cause any unfavorable reactions. Their use is wide-ranged: * In atmospheric chemistry, the most common scavenger is the hydroxyl radical, a short-lived radical produced photolytically in the atmosphere. It is the most important oxidant for carbon monoxide, methane and other hydrocarbons, sulfur dioxide, hydrogen sulfide, and most of other contaminants, removing them from the atmosphere. * In molecular laser isotope separation, methane is used as a scavenger gas for fluorine atoms. * Hydrazine and ascorbic acid are used as oxygen scavenger corrosion inhibitors. * Tocopherol and naringenin are bioactive free radical scavengers that act as antioxidants; synthetic catalytic scavengers are their synthetic counterparts * Organotin compounds are used in polymer manufacture as hydrochloric aci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |