|
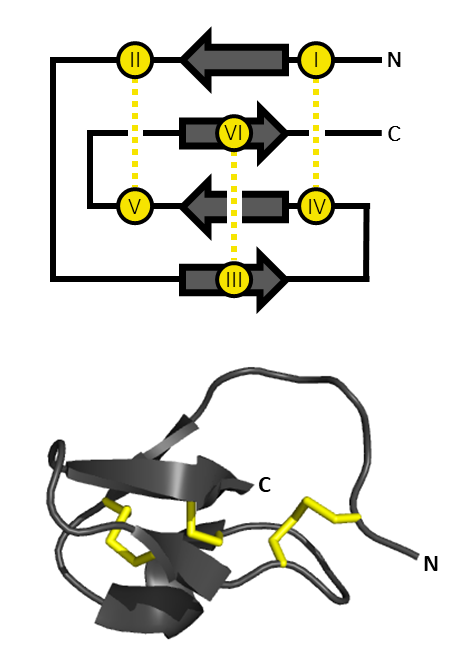
Ssm6a
Ssm6a (''Scolopendra subspinipes mutilans'' 6), or μ-SLPTX-Ssm6a, is a toxin from the venom of the Chinese red-headed centipede. It has strong analgesic properties, probably owing to its strong inhibitory effects on Nav1.7 channels. Sources Ssm6a is purified from the venom of the Chinese red-headed centipede, ''Scolopendra subspinipes mutilans'', found in Southeast Asia. Biochemistry Family Ssm6a is part of the scoloptoxin family (SPLTX), together with Ssm1a, Ssm2a, and Ssm3a, all found in the venom of the centipede. Synthesis The mature form of Ssm6a is composed of 46 amino acids. It is the result of posttranslational modification of a prepropeptide. The prepropeptide is 112 amino acids long. The 21 N-terminal amino acids are related to a signal sequence, the 43 following amino acids are referred as a propeptide sequence. The 46 C-terminal amino acids are the Ssm6a peptide. Structure Ssm6a shares only 40% of identity with its most related protein, κ-SLPTX-Ssm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chinese Red-headed Centipede
The Chinese red-headed centipede, also known as the Chinese red head, (''Scolopendra subspinipes mutilans'') is a centipede from East Asia and Australasia. It averages 20 cm (8 in) in length and lives in damp environments. In ancient Chinese traditions, this centipede is used for its healing properties. Putting a Chinese red head on a rash or other skin-disease is said to speed up the healing process. The roasted dry centipede is pulverized and used in Korea for the treatment of back pain, furuncles, and sores. ''S. s. mutilans'' is known for harbouring little aggression to other centipedes, a trait very rare amongst giant centipedes, and allows it to be kept communally. Antimicrobial activities of the identified compounds were reported against Gram-positive and Gram-negative bacteria, fungi, viruses, and parasites, that possibly explain centipede's survival in harsh and polluted environments. Females are incubator mothers, guarding the eggs by wrapping their ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Venom
Venom or zootoxin is a type of toxin produced by an animal that is actively delivered through a wound by means of a bite, sting, or similar action. The toxin is delivered through a specially evolved ''venom apparatus'', such as fangs or a stinger, in a process called envenomation. Venom is often distinguished from poison, which is a toxin that is passively delivered by being ingested, inhaled, or absorbed through the skin, and toxungen, which is actively transferred to the external surface of another animal via a physical delivery mechanism. Venom has evolved in terrestrial and marine environments and in a wide variety of animals: both predators and prey, and both vertebrates and invertebrates. Venoms kill through the action of at least four major classes of toxin, namely necrotoxins and cytotoxins, which kill cells; neurotoxins, which affect nervous systems; myotoxins, which damage muscles; and haemotoxins, which disrupt blood clotting. Venomous animals cause tens of thousa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Posttranslational Modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product. PTMs are important components in cell signal transduction, signaling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C-terminus, C- or N-terminus, N- termini. They can extend the chemical repertoire of the 20 standard amino acids by modifying an existing functional group or introducing a new one such as phosphorylation, phosphate. Phosphorylation is a highly effective mechanism for regulating the activity of enzymes and is the most common post-translational modification. Many eukaryotic and prokaryotic proteins also have carbohydra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prepropeptide
A protein precursor, also called a pro-protein or pro-peptide, is an inactive protein (or peptide) that can be turned into an active form by post-translational modification, such as breaking off a piece of the molecule or adding on another molecule. The name of the precursor for a protein is often prefixed by ''pro-''. Examples include proinsulin and proopiomelanocortin, which are both prohormones. Protein precursors are often used by an organism when the subsequent protein is potentially harmful, but needs to be available on short notice and/or in large quantities. Enzyme precursors are called zymogens or proenzymes. Examples are enzymes of the digestive tract in humans. Some protein precursors are secreted from the cell. Many of these are synthesized with an N-terminal signal peptide that targets them for secretion. Like other proteins that contain a signal peptide, their name is prefixed by ''pre''. They are thus called pre-pro-proteins or pre-pro-peptides. The signal peptide i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Signal Peptide
A signal peptide (sometimes referred to as signal sequence, targeting signal, localization signal, localization sequence, transit peptide, leader sequence or leader peptide) is a short peptide (usually 16-30 amino acids long) present at the N-terminus (or occasionally nonclassically at the C-terminus or internally) of most newly synthesized proteins that are destined toward the secretory pathway. These proteins include those that reside either inside certain organelles (the endoplasmic reticulum, Golgi or endosomes), secreted from the cell, or inserted into most cellular membranes. Although most type I membrane-bound proteins have signal peptides, the majority of type II and multi-spanning membrane-bound proteins are targeted to the secretory pathway by their first transmembrane domain, which biochemically resembles a signal sequence except that it is not cleaved. They are a kind of target peptide. Function (translocation) Signal peptides function to prompt a cell to translo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfide Bonds
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of protein, proteins. ''Persulfide'' usually refers to compounds. In inorganic chemistry disulfide usually refers to the corresponding anion (−S−S−). Organic disulfides Symmetrical disulfides are compounds of the formula . Most disulfides encountered in organo sulfur chemistry are symmetrical disulfides. Unsymmetrical disulfides (also called heterodisulfides) are compounds of the formula . They are less common in organic chemistry, but most disulfides in nature are unsymmetrical. Properties The disulfide bonds are strong, with a typical bond dissociation energy of 60 kcal/mol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inhibitor Cystine Knot
An inhibitor cystine knot (aka ICK or Knottin) is a protein structural motif containing three disulfide bridges. Knottins are one of three folds in the cystine knot motif; the other closely related knots are the Growth Factor Cystine Knot (GFCK) and the Cyclic Cystine Knot (CCK; cyclotide). Types include a) cyclic mobius, b) cyclic bracelet, c) acyclic inhibitor knottins. Cystine knot motifs are found frequently in nature in a plethora of plants, animals, and fungi and serve diverse functions from appetite suppression to anti-fungal activity. Along with the sections of polypeptide between them, two disulfides form a loop through which the third disulfide bond (linking the 3rd and 6th cysteine in the sequence) passes, forming a knot. The motif is common in invertebrate toxins such as those from arachnids and molluscs. The motif is also found in some inhibitor proteins found in plants, but the plant and animal motifs are thought to be a product of convergent evolution. The ICK moti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteases
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism (breakdown of old proteins), and cell signaling. In the absence of functional accelerants, proteolysis would be very slow, taking hundreds of years. Proteases can be found in all forms of life and viruses. They have independently evolved multiple times, and different classes of protease can perform the same reaction by completely different catalytic mechanisms. Hierarchy of proteases Based on catalytic residue Proteases can be classified into seven broad groups: * Serine proteases - ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IC50
The half maximal inhibitory concentration (IC50) is a measure of the potency of a substance in inhibiting a specific biological or biochemical function. IC50 is a quantitative measure that indicates how much of a particular inhibitory substance (e.g. drug) is needed to inhibit, ''in vitro'', a given biological process or biological component by 50%. The biological component could be an enzyme, cell, cell receptor or microorganism. IC50 values are typically expressed as molar concentration. IC50 is commonly used as a measure of antagonist drug potency in pharmacological research. IC50 is comparable to other measures of potency, such as EC50 for excitatory drugs. EC50 represents the dose or plasma concentration required for obtaining 50% of a maximum effect ''in vivo''. IC50 can be determined with functional assays or with competition binding assays. Sometimes, IC50 values are converted to the pIC50 scale. :\ce = -\log_ \ce Due to the minus sign, higher values of pIC50 indica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nociception
Nociception (also nocioception, from Latin ''nocere'' 'to harm or hurt') is the sensory nervous system's process of encoding noxious stimuli. It deals with a series of events and processes required for an organism to receive a painful stimulus, convert it to a molecular signal, and recognize and characterize the signal in order to trigger an appropriate defense response. In nociception, intense chemical (e.g., capsaicin present in Chili pepper or Cayenne pepper), mechanical (e.g., cutting, crushing), or thermal (heat and cold) stimulation of sensory neurons called nociceptors produces a signal that travels along a chain of nerve fibers via the spinal cord to the brain. Nociception triggers a variety of physiological and behavioral responses to protect the organism against an aggression and usually results in a subjective experience, or perception, of pain in sentient beings. Detection of noxious stimuli Potentially damaging mechanical, thermal, and chemical stimuli are detected ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Morphine
Morphine is a strong opiate that is found naturally in opium, a dark brown resin in poppies (''Papaver somniferum''). It is mainly used as a analgesic, pain medication, and is also commonly used recreational drug, recreationally, or to make other illicit drug, illicit opioids. There are numerous methods used to administer morphine: oral; sublingual administration, sublingual; via inhalation; intramuscular, injection into a muscle; by Subcutaneous injection, injection under the skin; intravenously; Intrathecally, injection into the space around the spinal cord; transdermal; or via rectal administration, rectal suppository. It acts directly on the central nervous system (CNS) to induce analgesia and alter perception and emotional response to pain. Physical and psychological dependence and tolerance may develop with repeated administration. It can be taken for both acute pain and chronic pain and is frequently used for pain from myocardial infarction, kidney stones, and during Ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |