|
Solid Lubricant
Dry lubricants or solid lubricants are materials that, despite being in the solid phase, are able to reduce friction between two surfaces sliding against each other without the need for a liquid oil medium. The two main dry lubricants are graphite and molybdenum disulfide. They offer lubrication at temperatures higher than liquid and oil-based lubricants operate. Dry lubricants are often used in applications such as locks or dry lubricated bearings. Such materials can operate up to 350 °C (662 °F) in oxidizing environments and even higher in reducing / non-oxidizing environments (molybdenum disulfide up to 1100 °C, 2012 °F). The low-friction characteristics of most dry lubricants are attributed to a layered structure on the molecular level with weak bonding between layers. Such layers are able to slide relative to each other with minimal applied force, thus giving them their low friction properties. However, a layered crystal structure alone is not necess ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Friction
Friction is the force resisting the relative motion of solid surfaces, fluid layers, and material elements sliding against each other. There are several types of friction: *Dry friction is a force that opposes the relative lateral motion of two solid surfaces in contact. Dry friction is subdivided into ''static friction'' ("stiction") between non-moving surfaces, and ''kinetic friction'' between moving surfaces. With the exception of atomic or molecular friction, dry friction generally arises from the interaction of surface features, known as asperities (see Figure 1). *Fluid friction describes the friction between layers of a viscous fluid that are moving relative to each other. *Lubricated friction is a case of fluid friction where a lubricant fluid separates two solid surfaces. *Skin friction is a component of drag, the force resisting the motion of a fluid across the surface of a body. *Internal friction is the force resisting motion between the elements making up a so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tungsten Disulfide
Tungsten disulfide is an inorganic chemical compound composed of tungsten and sulfur with the chemical formula WS2. This compound is part of the group of materials called the transition metal dichalcogenides. It occurs naturally as the rare mineral tungstenite. This material is a component of certain catalysts used for hydrodesulfurization and hydrodenitrification. WS2 adopts a layered structure similar, or isotypic with MoS2, instead with W atoms situated in trigonal prismatic coordination sphere (in place of Mo atoms). Owing to this layered structure, WS2 forms non-carbon nanotubes, which were discovered after heating a thin sample of WS2 in 1992. Structure and physical properties Bulk WS2 forms dark gray hexagonal crystals with a layered structure. Like the closely related MoS2, it exhibits properties of a dry lubricant. Although it has long been thought that WS2 is relatively stable in ambient air, recent reports on the ambient air oxidation of monolayer WS2 have found this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nylon
Nylon is a generic designation for a family of synthetic polymers composed of polyamides ( repeating units linked by amide links).The polyamides may be aliphatic or semi-aromatic. Nylon is a silk-like thermoplastic, generally made from petroleum, that can be melt-processed into fibers, films, or shapes. Nylon polymers can be mixed with a wide variety of additives to achieve many property variations. Nylon polymers have found significant commercial applications in fabric and fibers (apparel, flooring and rubber reinforcement), in shapes (molded parts for cars, electrical equipment, etc.), and in films (mostly for food packaging). History DuPont and the invention of nylon Researchers at DuPont began developing cellulose based fibers, culminating in the synthetic fiber rayon. DuPont's experience with rayon was an important precursor to its development and marketing of nylon. DuPont's invention of nylon spanned an eleven-year period, ranging from the initial research pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-ring
An O-ring, also known as a packing or a toric joint, is a mechanical gasket in the shape of a torus; it is a loop of elastomer with a round cross-section, designed to be seated in a groove and compressed during assembly between two or more parts, forming a seal at the interface. The O-ring may be used in static applications or in dynamic applications where there is relative motion between the parts and the O-ring. Dynamic examples include rotating pump shafts and hydraulic cylinder pistons. Static applications of O-rings may include fluid or gas sealing applications in which: (1) the O-ring is compressed resulting in zero clearance, (2) the O-ring material is vulcanized solid such that it is impermeable to the fluid or gas, and (3) the O-ring material is resistant to degradation by the fluid or gas. The wide range of potential liquids and gases that need to be sealed has necessitated the development of a wide range of materials. O-rings are one of the most common seals used i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Universal Joint
A universal joint (also called a universal coupling or U-joint) is a joint or coupling connecting rigid shafts whose axes are inclined to each other. It is commonly used in shafts that transmit rotary motion. It consists of a pair of hinges located close together, oriented at 90° to each other, connected by a cross shaft. The universal joint is not a constant-velocity joint. U-joints are also sometimes called by various eponymous names, as follows: * Cardan joint, after Gerolamo Cardano, a polymath of the 16th century who contributed to knowledge of various clever mechanisms, including gimbals * Hooke joint or Hooke's joint, after Robert Hooke, a polymath of the 17th century who contributed to knowledge of various clever mechanisms * Spicer joint, after Clarence W. Spicer and the Spicer Manufacturing Company, who manufactured U joints * Hardy Spicer joint, after the Hardy Spicer brand, a successor to the Spicer brand History The main concept of the universal joint is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spline (mechanical)
Splines are ridges or teeth on a drive shaft that matches with grooves in a mating piece and transfer torque to it, maintaining the angular correspondence between them. For instance, a gear mounted on a shaft might use a male spline on the shaft that matches the female spline on the gear. The splines on the pictured drive shaft match with the female splines in the center of the clutch plate, while the smooth tip of the axle is supported in the pilot bearing in the flywheel. An alternative to splines is a keyway and key, though splines provide a longer fatigue life, and can carry significantly greater torques for the size. Types There are several types of splines: ;Parallel key spline: where the sides of the equally spaced grooves are parallel in both directions, radial and axial. ;Involute spline: where the sides of the equally spaced grooves are involute, as with an involute gear, but not as tall. The curves increase strength by decreasing stress concentrations. ;Crowned spl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Energy
In surface science, surface free energy (also interfacial free energy or surface energy) quantifies the disruption of intermolecular bonds that occurs when a surface is created. In solid-state physics, surfaces must be intrinsically less energetically favorable than the bulk of the material (the atoms on the surface have more energy compared with the atoms in the bulk), otherwise there would be a driving force for surfaces to be created, removing the bulk of the material (see sublimation). The surface energy may therefore be defined as the excess energy at the surface of a material compared to the bulk, or it is the work required to build an area of a particular surface. Another way to view the surface energy is to relate it to the work required to cut a bulk sample, creating two surfaces. There is "excess energy" as a result of the now-incomplete, unrealized bonding at the two surfaces. Cutting a solid body into pieces disrupts its bonds and increases the surface area, and th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polytetrafluorethylene
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene that has numerous applications. It is one of the best-known and widely applied PFAS. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a spin-off from DuPont, which originally discovered the compound in 1938. Polytetrafluoroethylene is a fluorocarbon solid, as it is a high-molecular-weight polymer consisting wholly of carbon and fluorine. PTFE is hydrophobic: neither water nor water-containing substances wet PTFE, as fluorocarbons exhibit only small London dispersion forces due to the low electric polarizability of fluorine. PTFE has one of the lowest coefficients of friction of any solid. Polytetrafluoroethylene is used as a non-stick coating for pans and other cookware. It is non-reactive, partly because of the strength of carbon–fluorine bonds, so it is often used in containers and pipework for reactive and corrosive chemicals. Where used as a lubricant, PTFE ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
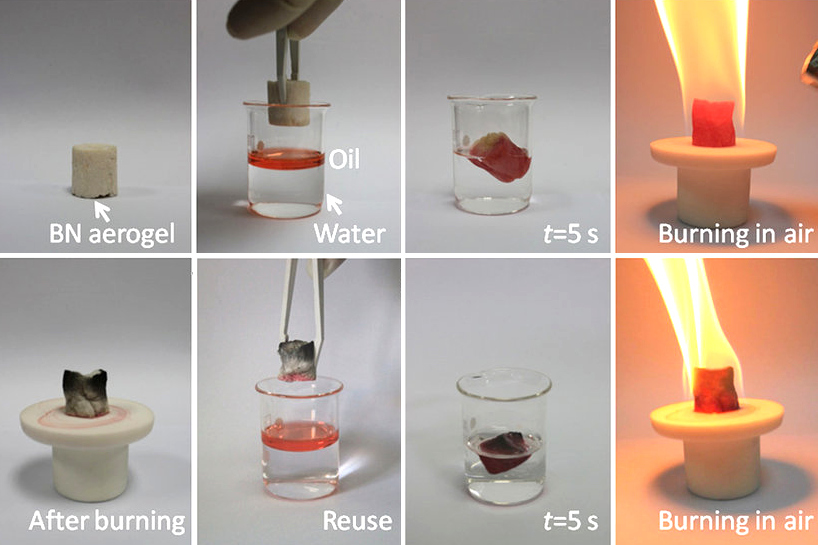
Boron Nitride
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic ( zincblende aka sphalerite structure) variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form. Because of excellent thermal and chemical stability, boron nitride ceramics are used in high-temperature equipment and metal casting. Boron nitride has potential use in nanotechnology. Structure Boron nitride exists in multiple forms that differ in the arrangement of the boron and nitrogen atoms, giving rise to varyin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdenum Disulfide
Molybdenum disulfide (or moly) is an inorganic compound composed of molybdenum and sulfur. Its chemical formula is . The compound is classified as a transition metal dichalcogenide. It is a silvery black solid that occurs as the mineral molybdenite, the principal ore for molybdenum.Sebenik, Roger F. ''et al''. (2005) "Molybdenum and Molybdenum Compounds", ''Ullmann's Encyclopedia of Chemical Technology''. Wiley-VCH, Weinheim. is relatively unreactive. It is unaffected by dilute acids and oxygen. In appearance and feel, molybdenum disulfide is similar to graphite. It is widely used as a dry lubricant because of its low friction and robustness. Bulk is a diamagnetic, indirect bandgap semiconductor similar to silicon, with a bandgap of 1.23 eV. Single layer sheets act as a perfect mirror, reflecting 100% of incident photons. Production MoS2 is naturally found as either molybdenite, a crystalline mineral, or jordisite, a rare low temperature form of molybdenite. Molybdenite ore is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sintering
Clinker nodules produced by sintering Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction. Sintering happens as part of a manufacturing process used with metals, ceramics, plastics, and other materials. The atoms in the materials diffuse across the boundaries of the particles, fusing the particles together and creating one solid piece. Because the sintering temperature does not have to reach the melting point of the material, sintering is often chosen as the shaping process for materials with extremely high melting points such as tungsten and molybdenum. The study of sintering in metallurgical powder-related processes is known as powder metallurgy. An example of sintering can be observed when ice cubes in a glass of water adhere to each other, which is driven by the temperature difference between the water and the ice. Examples of pressure-driven sintering are the compact ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large scale (300 kton/year, in 1989) for uses in pencils, lubricants, and electrodes. Under high pressures and temperatures it converts to diamond. It is a weak conductor of heat and electricity. Types and varieties Natural graphite The principal types of natural graphite, each occurring in different types of ore deposits, are * Crystalline small flakes of graphite (or flake graphite) occurs as isolated, flat, plate-like particles with hexagonal edges if unbroken. When broken the edges can be irregular or angular; * Amorphous graphite: very fine flake graphite is sometimes called amorphous; * Lump graphite (or vein graphite) occurs in fissure veins or fractures and appears as massive platy intergrowths of fibrous or acicular crystalline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |