|
Solanidine To 16-DPA Conversion
Solanidine is a poisonous steroidal alkaloid chemical compound that occurs in plants of the family Solanaceae, such as potato and ''Solanum americanum''. Human ingestion of solanidine also occurs via the consumption of the glycoalkaloids, α-solanine and α-chaconine, present in potatoes.Kuiper-Goodman, T., Nawrot, P.S.Solanine and Chaconine IPCS Inchem The sugar portion of these glycoalkaloids hydrolyses in the body, leaving the solanidine portion. Solanidine occurs in the blood serum of normal healthy people who eat potato, and serum solanidine levels fall markedly once potato consumption ceases. Solanidine from food is also stored in the human body for prolonged periods of time, and it has been suggested that it could be released during times of metabolic stress with the potential for deleterious consequences. Solanidine is responsible for neuromuscular syndromes via cholinesterase inhibition.Everist, S.L., ''Poisonous Plants of Australia'', Angus and Robertson, 1974, . Us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Poisonous
Poison is a chemical substance that has a detrimental effect to life. The term is used in a wide range of scientific fields and industries, where it is often specifically defined. It may also be applied colloquially or figuratively, with a broad sense. Whether something is considered a poison may change depending on the amount, the circumstances, and what living things are present. Poisoning could be accidental or deliberate, and if the cause can be identified there may be ways to neutralise the effects or minimise the symptoms. In biology, a poison is a chemical substance causing death, injury or harm to organisms or their parts. In medicine, poisons are a kind of toxin that are delivered passively, not actively. In industry the term may be negative, something to be removed to make a thing safe, or positive, an agent to limit unwanted pests. In ecological terms, poisons introduced into the environment can later cause unwanted effects elsewhere, or in other parts of the food ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hormones
A hormone (from the Ancient Greek, Greek participle , "setting in motion") is a class of cell signaling, signaling molecules in multicellular organisms that are sent to distant organs by complex biological processes to regulate physiology and behavior. Hormones are required for the correct development of animals, plants and fungi. Due to the broad definition of a hormone (as a signaling molecule that exerts its effects far from its site of production), numerous kinds of molecules can be classified as hormones. Among the substances that can be considered hormones, are eicosanoids (e.g. prostaglandins and thromboxanes), steroids (e.g. Estrogen, oestrogen and brassinosteroid), amino acid derivatives (e.g. epinephrine and auxin), protein or peptides (e.g. insulin and CLE peptides), and gases (e.g. ethylene and nitric oxide). Hormones are used to communicate between organ (anatomy), organs and Tissue (biology), tissues. In vertebrates, hormones are responsible for regulating a variety ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury(II) Acetate
Mercury(II) acetate is the chemical compound with the formula Hg( O2 CC H3)2. Commonly abbreviated Hg(OAc)2, this compound is employed as a reagent to generate organomercury compounds from unsaturated organic precursors. It is a white water-soluble solid, but samples appear yellowish with time owing to decomposition. Structure Mercury(II) acetate is a crystalline solid consisting of isolated Hg(OAc)2 molecules with Hg-O distances of 2.07 Å. Three long, weak intermolecular Hg···O bonds of about 2.75 Å are also present, resulting in a slightly distorted square pyramidal coordination geometry at Hg. Synthesis and reactions Mercury(II) acetate can be produced by reaction of mercuric oxide with acetic acid. Inorganic reactions Mercury(II) acetate in acetic acid solution reacts with H2S to rapidly precipitate the black (β) polymorph of HgS. With gentle heating of the slurry, the black solid converts to the red form. The mineral cinnabar is red HgS. The precipita ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solanidine To 16-DPA Conversion
Solanidine is a poisonous steroidal alkaloid chemical compound that occurs in plants of the family Solanaceae, such as potato and ''Solanum americanum''. Human ingestion of solanidine also occurs via the consumption of the glycoalkaloids, α-solanine and α-chaconine, present in potatoes.Kuiper-Goodman, T., Nawrot, P.S.Solanine and Chaconine IPCS Inchem The sugar portion of these glycoalkaloids hydrolyses in the body, leaving the solanidine portion. Solanidine occurs in the blood serum of normal healthy people who eat potato, and serum solanidine levels fall markedly once potato consumption ceases. Solanidine from food is also stored in the human body for prolonged periods of time, and it has been suggested that it could be released during times of metabolic stress with the potential for deleterious consequences. Solanidine is responsible for neuromuscular syndromes via cholinesterase inhibition.Everist, S.L., ''Poisonous Plants of Australia'', Angus and Robertson, 1974, . Us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
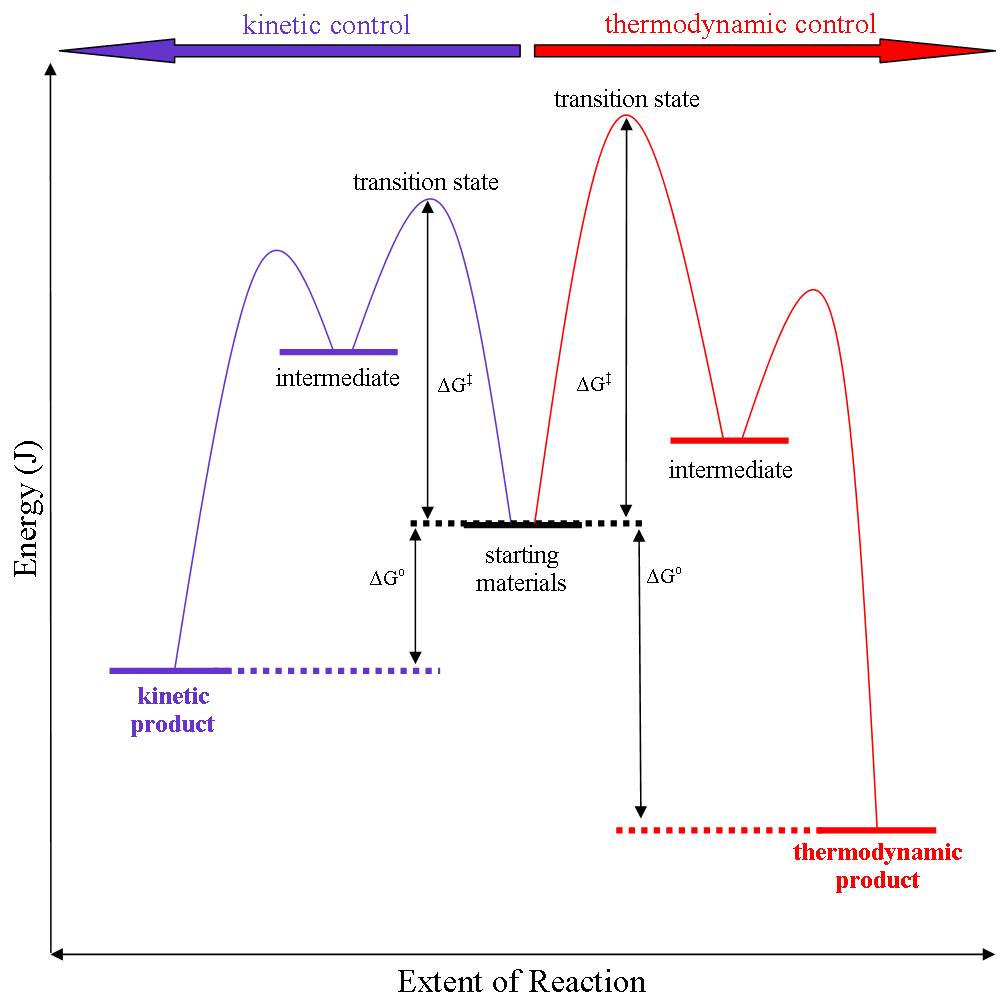
Thermodynamic Versus Kinetic Reaction Control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the conversion (chemistry), selectivity or stereoselectivity. The distinction is relevant when product A forms faster than product B because the activation energy for product A is lower than that for product B, yet product B is more stable. In such a case A is the kinetic product and is favoured under kinetic control and B is the thermodynamic product and is favoured under thermodynamic control.Introduction to Organic Chemistry I, Seth Robert Elsheimer, Blackwell Publishing, 2000 The conditions of the reaction, such as temperature, pressure, or solvent, affect which reaction pathway may be favored: either the kinetically controlled or the thermodynamically controlled one. Note this is only true if the activation energy of the two pathways differ, with one p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iminium
In organic chemistry, an iminium cation is a polyatomic ion with the general structure . They are common in synthetic chemistry and biology. Structure Iminium cations adopt alkene-like geometries. The central C=N unit is nearly coplanar with all four substituents. The C=N distances, which are near 129 picometers in length, are shorter than C-N single bonds. Cis/trans isomers are observed. Formation Iminium cations are obtained by protonation and alkylation of imines: :RN=CR'_2 + H+ -> NH=CR'_2 :RN=CR'_2 + R''+ -> R''N=CR'_2 They also are generated by the condensation of secondary amines with ketones or aldehydes: :O=CR'_2 + R2NH + H+ 2N=CR'_2 + H2O This rapid, reversible reaction is one step in "iminium catalysis". More exotic routes to iminium cations are known, e.g. from ring-opening reactions of pyridine. Occurrence Iminium derivatives are common in biology. Pyridoxal phosphate reacts with amino acids to give iminium derivatives. Many iminium salts are en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iminium
In organic chemistry, an iminium cation is a polyatomic ion with the general structure . They are common in synthetic chemistry and biology. Structure Iminium cations adopt alkene-like geometries. The central C=N unit is nearly coplanar with all four substituents. The C=N distances, which are near 129 picometers in length, are shorter than C-N single bonds. Cis/trans isomers are observed. Formation Iminium cations are obtained by protonation and alkylation of imines: :RN=CR'_2 + H+ -> NH=CR'_2 :RN=CR'_2 + R''+ -> R''N=CR'_2 They also are generated by the condensation of secondary amines with ketones or aldehydes: :O=CR'_2 + R2NH + H+ 2N=CR'_2 + H2O This rapid, reversible reaction is one step in "iminium catalysis". More exotic routes to iminium cations are known, e.g. from ring-opening reactions of pyridine. Occurrence Iminium derivatives are common in biology. Pyridoxal phosphate reacts with amino acids to give iminium derivatives. Many iminium salts are en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour. Acetone is miscible with water and serves as an important organic solvent in its own right, in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and production of methyl methacrylate (and from that PMMA) as well as bisphenol A.Acetone World Petrochemicals report, January 2010Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. It is a common building block in |
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide. Properties Physical properties The molecular electric dipole moment is 2.2 debyes. Pyridine is diamagnetic and has a diamagnetic susceptibility of −48.7 × 10−6 cm3·mol−1. The standard enthalpy of formation is 100.2 kJ·mol−1 in the liquid phase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylene Chloride
Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with water, it is slightly polar, and miscible with many organic solvents.Rossberg, M. ''et al.'' (2006) "Chlorinated Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. . Occurrence Natural sources of dichloromethane include oceanic sources, macroalgae, wetlands, and volcanoes. However, the majority of dichloromethane in the environment is the result of industrial emissions. Production DCM is produced by treating either chloromethane or methane with chlorine gas at 400–500 °C. At these temperatures, both methane and chloromethane undergo a series of reactions producing progressively more chlorinated products. In this way, an estimated 400,000 tons were produced in the US, Europe, and Japan in 1993. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not classed as organic). It is produced mainly as a byproduct of acrylonitrile manufacture. It is used as a polar aprotic solvent in organic synthesis and in the purification of butadiene. The skeleton is linear with a short distance of 1.16 Å. Acetonitrile was first prepared in 1847 by the French chemist Jean-Baptiste Dumas. Applications Acetonitrile is used mainly as a solvent in the purification of butadiene in refineries. Specifically, acetonitrile is fed into the top of a distillation column filled with hydrocarbons including butadiene, and as the acetonitrile falls down through the column, it absorbs the butadiene which is then sent from the bottom of the tower to a second separating tower. Heat is then employed in the separatin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemical
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outcome of a particular chemical change, or vice versa. These reactions involve electrons moving via an electronically-conducting phase (typically an external electrical circuit, but not necessarily, as in electroless plating) between electrodes separated by an ionically conducting and electronically insulating electrolyte (or ionic species in a solution). When a chemical reaction is driven by an electrical potential difference, as in electrolysis, or if a potential difference results from a chemical reaction as in an electric battery or fuel cell, it is called an ''electrochemical'' reaction. Unlike in other chemical reactions, in electrochemical reactions electrons are not transferred directly between atoms, ions, or molecules, but via the af ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |