|
Slater Determinant
In quantum mechanics, a Slater determinant is an expression that describes the wave function of a multi-fermionic system. It satisfies anti-symmetry requirements, and consequently the Pauli principle, by changing sign upon exchange of two electrons (or other fermions).Molecular Quantum Mechanics Parts I and II: An Introduction to QUANTUM CHEMISTRY (Volume 1), P. W. Atkins, Oxford University Press, 1977, . Only a small subset of all possible fermionic wave functions can be written as a single Slater determinant, but those form an important and useful subset because of their simplicity. The Slater determinant arises from the consideration of a wave function for a collection of electrons, each with a wave function known as the spin-orbital \chi(\mathbf), where \mathbf denotes the position and spin of a single electron. A Slater determinant containing two electrons with the same spin orbital would correspond to a wave function that is zero everywhere. The Slater determinant is named ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, quantum field theory, quantum technology, and quantum information science. Classical physics, the collection of theories that existed before the advent of quantum mechanics, describes many aspects of nature at an ordinary (macroscopic) scale, but is not sufficient for describing them at small (atomic and subatomic) scales. Most theories in classical physics can be derived from quantum mechanics as an approximation valid at large (macroscopic) scale. Quantum mechanics differs from classical physics in that energy, momentum, angular momentum, and other quantities of a bound system are restricted to discrete values ( quantization); objects have characteristics of both particles and waves (wave–particle duality); and there are limits to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linearly Dependent
In the theory of vector spaces, a set (mathematics), set of vector (mathematics), vectors is said to be if there is a nontrivial linear combination of the vectors that equals the zero vector. If no such linear combination exists, then the vectors are said to be . These concepts are central to the definition of Dimension (vector space), dimension. A vector space can be of finite dimension or infinite dimension depending on the maximum number of linearly independent vectors. The definition of linear dependence and the ability to determine whether a subset of vectors in a vector space is linearly dependent are central to determining the dimension of a vector space. Definition A sequence of vectors \mathbf_1, \mathbf_2, \dots, \mathbf_k from a vector space is said to be ''linearly dependent'', if there exist Scalar (mathematics), scalars a_1, a_2, \dots, a_k, not all zero, such that :a_1\mathbf_1 + a_2\mathbf_2 + \cdots + a_k\mathbf_k = \mathbf, where \mathbf denotes the z ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hund's Rule
Hund's rule of maximum multiplicity is a rule based on observation of atomic spectra, which is used to predict the ground state of an atom or molecule with one or more open electronic shells. The rule states that for a given electron configuration, the lowest energy term is the one with the greatest value of spin multiplicity. This implies that if two or more orbitals of equal energy are available, electrons will occupy them singly before filling them in pairs. The rule, discovered by Friedrich Hund in 1925, is of important use in atomic chemistry, spectroscopy, and quantum chemistry, and is often abbreviated to Hund's rule, ignoring Hund's other two rules. Atoms The multiplicity of a state is defined as 2S + 1, where S is the total electronic spin. A high multiplicity state is therefore the same as a high-spin state. The lowest-energy state with maximum multiplicity usually has unpaired electrons all with parallel spin. Since the spin of each electron is 1/2, the total spin is on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physical Chemistry
Physical chemistry is the study of macroscopic and microscopic phenomena in chemical systems in terms of the principles, practices, and concepts of physics such as motion, energy, force, time, thermodynamics, quantum chemistry, statistical mechanics, analytical dynamics and chemical equilibria. Physical chemistry, in contrast to chemical physics, is predominantly (but not always) a supra-molecular science, as the majority of the principles on which it was founded relate to the bulk rather than the molecular or atomic structure alone (for example, chemical equilibrium and colloids). Some of the relationships that physical chemistry strives to resolve include the effects of: # Intermolecular forces that act upon the physical properties of materials ( plasticity, tensile strength, surface tension in liquids). # Reaction kinetics on the rate of a reaction. # The identity of ions and the electrical conductivity of materials. # Surface science and electrochemistry of cell membrane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, quantum field theory, quantum technology, and quantum information science. Classical physics, the collection of theories that existed before the advent of quantum mechanics, describes many aspects of nature at an ordinary (macroscopic) scale, but is not sufficient for describing them at small (atomic and subatomic) scales. Most theories in classical physics can be derived from quantum mechanics as an approximation valid at large (macroscopic) scale. Quantum mechanics differs from classical physics in that energy, momentum, angular momentum, and other quantities of a bound system are restricted to discrete values ( quantization); objects have characteristics of both particles and waves (wave–particle duality); and there are limits to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Electrodynamics
In particle physics, quantum electrodynamics (QED) is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved. QED mathematically describes all phenomena involving electrically charged particles interacting by means of exchange of photons and represents the quantum counterpart of classical electromagnetism giving a complete account of matter and light interaction. In technical terms, QED can be described as a perturbation theory of the electromagnetic quantum vacuum. Richard Feynman called it "the jewel of physics" for its extremely accurate predictions of quantities like the anomalous magnetic moment of the electron and the Lamb shift of the energy levels of hydrogen. History The first formulation of a quantum theory describing radiation and matter interaction is attributed to British scientist Paul Dirac, who ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fock Space
The Fock space is an algebraic construction used in quantum mechanics to construct the quantum states space of a variable or unknown number of identical particles from a single particle Hilbert space . It is named after V. A. Fock who first introduced it in his 1932 paper "Konfigurationsraum und zweite Quantelung" (" Configuration space and second quantization"). M.C. Reed, B. Simon, "Methods of Modern Mathematical Physics, Volume II", Academic Press 1975. Page 328. Informally, a Fock space is the sum of a set of Hilbert spaces representing zero particle states, one particle states, two particle states, and so on. If the identical particles are bosons, the -particle states are vectors in a symmetrized tensor product of single-particle Hilbert spaces . If the identical particles are fermions, the -particle states are vectors in an antisymmetrized tensor product of single-particle Hilbert spaces (see symmetric algebra and exterior algebra respectively). A general state in Foc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
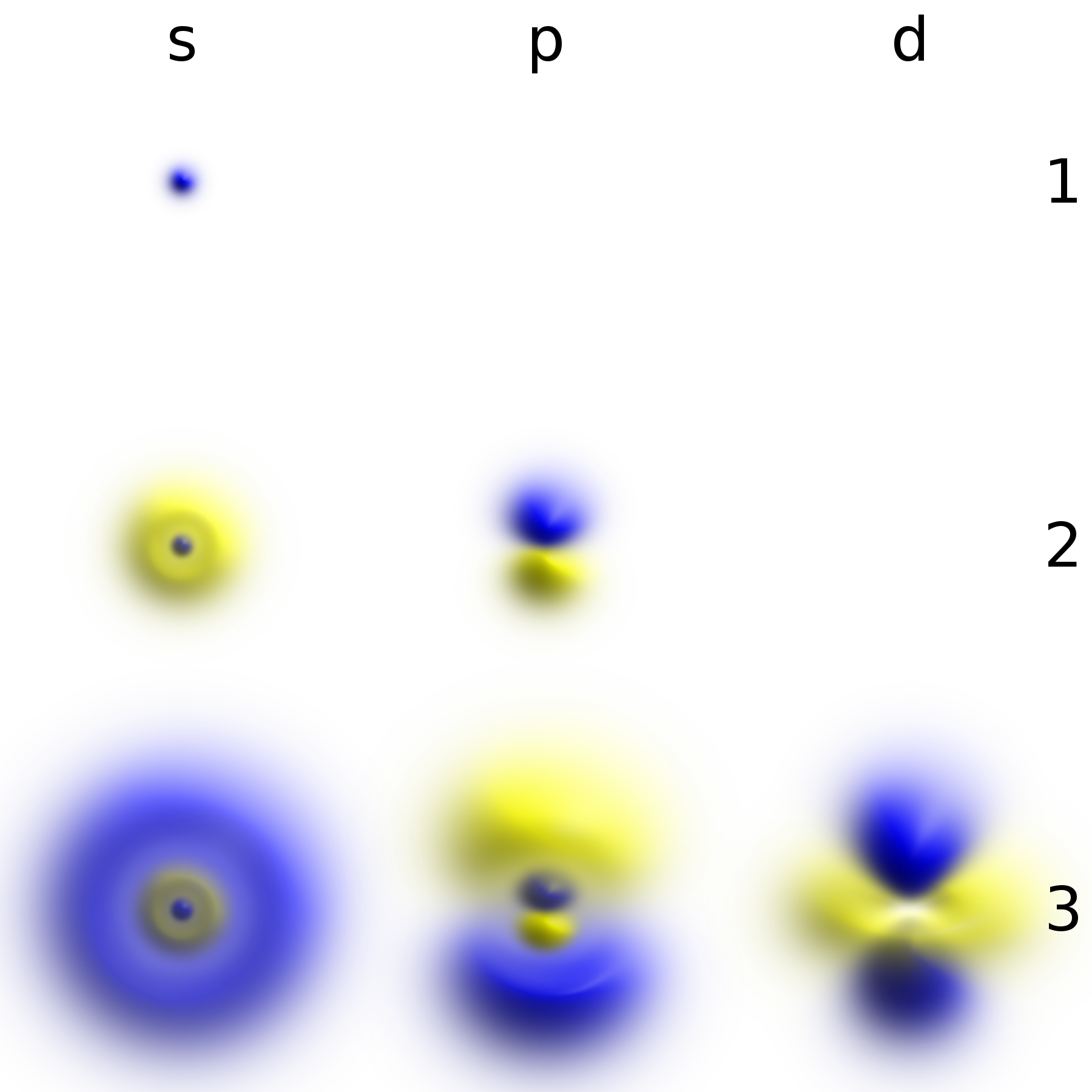
Atomic Orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term ''atomic orbital'' may also refer to the physical region or space where the electron can be calculated to be present, as predicted by the particular mathematical form of the orbital. Each orbital in an atom is characterized by a set of values of the three quantum numbers , , and , which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (magnetic quantum number). Alternative to the magnetic quantum number, the orbitals are often labeled by the associated harmonic polynomials (e.g., ''xy'', ). Each such orbital can be occupied by a maximum of two electrons, each with its own projection of spin m_s. The simple names s orbital, p orb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antisymmetrizer
In quantum mechanics, an antisymmetrizer \mathcal (also known as antisymmetrizing operatorP.A.M. Dirac, ''The Principles of Quantum Mechanics'', 4th edition, Clarendon, Oxford UK, (1958) p. 248) is a linear operator that makes a wave function of ''N'' identical fermions antisymmetric under the exchange of the coordinates of any pair of fermions. After application of \mathcal the wave function satisfies the Pauli exclusion principle. Since \mathcal is a projection operator, application of the antisymmetrizer to a wave function that is already totally antisymmetric has no effect, acting as the identity operator. Mathematical definition Consider a wave function depending on the space and spin coordinates of ''N'' fermions: : \Psi(1,2, \ldots, N)\quad\text \quad i \leftrightarrow (\mathbf_i, \sigma_i), where the position vector r''i'' of particle ''i'' is a vector in \mathbb^3 and σi takes on 2''s''+1 values, where ''s'' is the half-integral intrinsic spin of the fermion. For ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Permanent (mathematics)
In linear algebra, the permanent of a square matrix is a function of the matrix similar to the determinant. The permanent, as well as the determinant, is a polynomial in the entries of the matrix. Both are special cases of a more general function of a matrix called the immanant. Definition The permanent of an matrix is defined as \operatorname(A)=\sum_\prod_^n a_. The sum here extends over all elements σ of the symmetric group ''S''''n''; i.e. over all permutations of the numbers 1, 2, ..., ''n''. For example, \operatorname\begina&b \\ c&d\end=ad+bc, and \operatorname\begina&b&c \\ d&e&f \\ g&h&i \end=aei + bfg + cdh + ceg + bdi + afh. The definition of the permanent of ''A'' differs from that of the determinant of ''A'' in that the signatures of the permutations are not taken into account. The permanent of a matrix A is denoted per ''A'', perm ''A'', or Per ''A'', sometimes with parentheses around the argument. Minc uses Per(''A'') for the permanent of rectangular mat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bosons
In particle physics, a boson ( ) is a subatomic particle whose spin quantum number has an integer value (0,1,2 ...). Bosons form one of the two fundamental classes of subatomic particle, the other being fermions, which have odd half-integer spin (,, ...). Every observed subatomic particle is either a boson or a fermion. Bosons are named after physicist Satyendra Nath Bose. Some bosons are elementary particles and occupy a special role in particle physics unlike that of fermions, which are sometimes described as the constituents of "ordinary matter". Some elementary bosons (for example, gluons) act as force carriers, which give rise to forces between other particles, while one (the Higgs boson) gives rise to the phenomenon of mass. Other bosons, such as mesons, are composite particles made up of smaller constituents. Outside the realm of particle physics, superfluidity arises because composite bosons (bose particles), such as low temperature helium-4 atoms, follow Bose–Einst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MCSCF
Multi-configurational self-consistent field (MCSCF) is a method in quantum chemistry used to generate qualitatively correct reference states of molecules in cases where Hartree–Fock and density functional theory are not adequate (e.g., for molecular ground states which are quasi-degenerate with low-lying excited states or in bond-breaking situations). It uses a linear combination of configuration state functions (CSF), or configuration determinants, to approximate the exact electronic wavefunction of an atom or molecule. In an MCSCF calculation, the set of coefficients of both the CSFs or determinants and the basis functions in the molecular orbitals are varied to obtain the total electronic wavefunction with the lowest possible energy. This method can be considered a combination between configuration interaction (where the molecular orbitals are not varied but the expansion of the wave function) and Hartree–Fock (where there is only one determinant, but the molecular orbitals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |