|
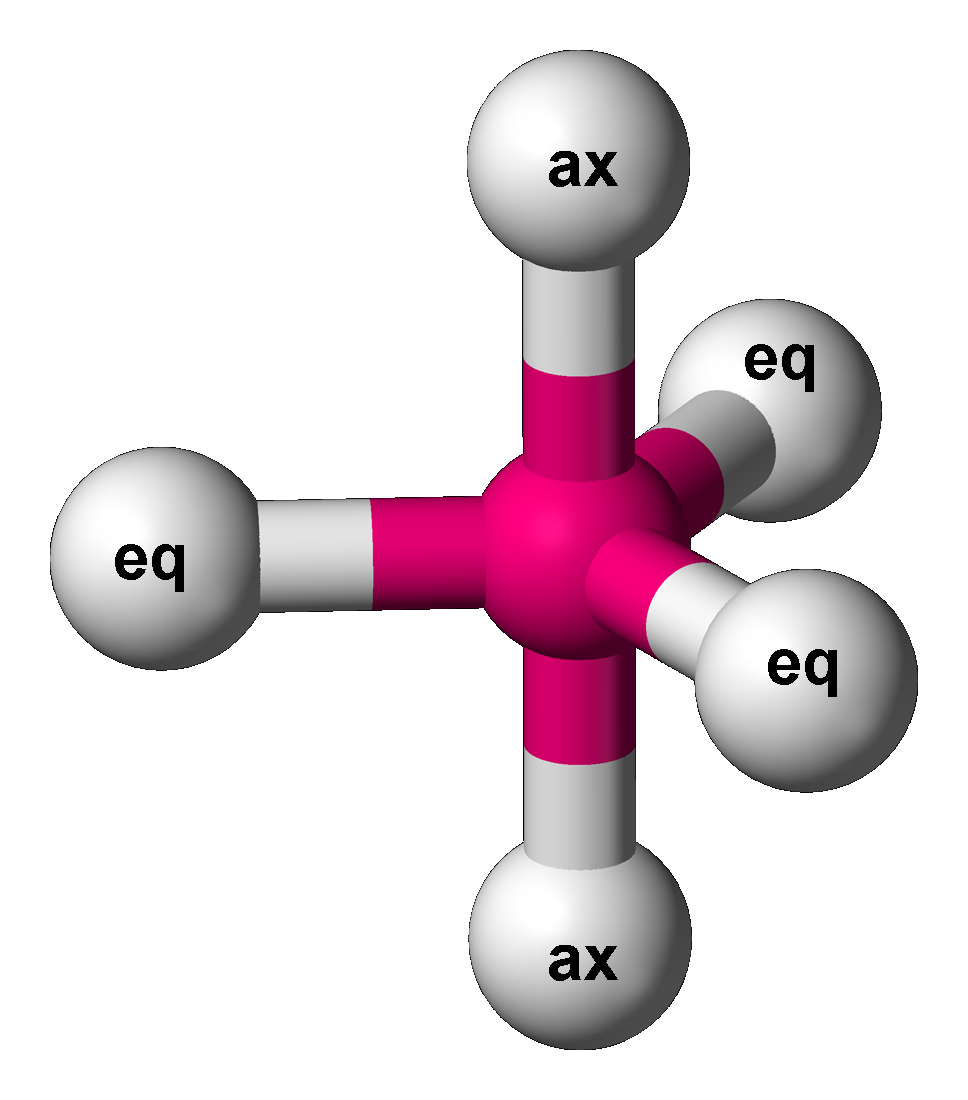
Seesaw (chemistry)
Disphenoidal or seesaw (also known as sawhorse) is a type of molecular geometry where there are four bonds to a central atom with overall C2v molecular symmetry. The name "seesaw" comes from the observation that it looks like a playground seesaw. Most commonly, four bonds to a central atom result in tetrahedral or, less commonly, square planar geometry. The seesaw geometry occurs when a molecule has a steric number of 5, with the central atom being bonded to 4 other atoms and 1 lone pair (AX4E1 in AXE notation). An atom bonded to 5 other atoms (and no lone pairs) forms a trigonal bipyramid with two axial and three equatorial positions, but in the seesaw geometry one of the atoms is replaced by a lone pair of electrons, which is always in an equatorial position. This is true because the lone pair occupies more space near the central atom (A) than does a bonding pair of electrons. An equatorial lone pair is repelled by only two bonding pairs at 90°, whereas a hypothetical axial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Symmetry In Three Dimensions
In three dimensional geometry, there are four infinite series of point groups in three dimensions (''n''≥1) with ''n''-fold rotational or reflectional symmetry about one axis (by an angle of 360°/''n'') that does not change the object. They are the finite symmetry groups on a cone. For ''n'' = ∞ they correspond to four frieze groups. Schönflies notation is used. The terms horizontal (h) and vertical (v) imply the existence and direction of reflections with respect to a vertical axis of symmetry. Also shown are Coxeter notation in brackets, and, in parentheses, orbifold notation. Types ;Chiral: *''Cn'', sup>+, (''nn'') of order ''n'' - ''n''-fold rotational symmetry - acro-n-gonal group (abstract group ''Zn''); for ''n''=1: no symmetry (trivial group) ;Achiral: *''Cnh'', +,2 (''n''*) of order 2''n'' - prismatic symmetry or ortho-n-gonal group (abstract group ''Zn'' × ''Dih1''); for ''n''=1 this is denoted by ''Cs'' (1*) and called reflection symmetry, also bilateral ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
VSEPR Theory
Valence shell electron pair repulsion (VSEPR) theory ( , ), is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Nyholm. The premise of VSEPR is that the valence electron pairs surrounding an atom tend to repel each other and will, therefore, adopt an arrangement that minimizes this repulsion. This in turn decreases the molecule's energy and increases its stability, which determines the molecular geometry. Gillespie has emphasized that the electron-electron repulsion due to the Pauli exclusion principle is more important in determining molecular geometry than the electrostatic repulsion. The insights of VSEPR theory are derived from topological analysis of the electron density of molecules. Such quantum chemical topology (QCT) methods include the electron localization function (ELF) and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Geometry
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism and biological activity. The angles between bonds that an atom forms depend only weakly on the rest of molecule, i.e. they can be understood as approximately local and hence transferable properties. Determination The molecular geometry can be determined by various spectroscopic methods and diffraction methods. IR, microwave and Raman spectroscopy can give information about the molecule geometry from the details of the vibrational and rotational absorbance detected by these techniques. X-ray crystallography, neutron diffraction and electron diffraction can give molecular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Tetrachloride
Sulfur tetrachloride is an inorganic compound with chemical formula SCl4. It has only been obtained as an unstable pale yellow solid. The corresponding SF4 is a stable, useful reagent. Preparation and structure It is obtained by treating sulfur dichloride with chlorine at 193 K: It melts with simultaneous decomposition above −20 °C.Georg Brauer: ''Handbuch der Präparativen Anorganischen Chemie''. Its solid structure is uncertain. It is probably the salt SCl3+Cl−, since related salts are known with noncoordinating anions. In contrast to this tetrachloride, SF4 is a neutral molecule.Goettel, J. T., Kostiuk, N. and Gerken, M. (2013), ''The Solid-State Structure of SF4: The Final Piece of the Puzzle'' . Angew. Chem. Int. Ed., 52: 8037–8040. Reactions It decomposes above −30 °C (242 K) to sulfur dichloride and chlorine. It hydrolyzes readily: Sulfur tetrachloride reacts with water, producing hydrogen chloride and sulfur dioxide through the hydrolysis proce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xenon Dioxydifluoride
Xenon dioxydifluoride is an inorganic chemical compound with the formula XeO2F2. At room temperature it exists as a metastable solid, which decomposes slowly into xenon difluoride, but the cause of this decomposition is unknown. Preparation Xenon dioxydifluoride is prepared by reacting xenon trioxide with xenon oxytetrafluoride Xenon oxytetrafluoride () is an inorganic chemical compound. It is a colorless stable liquid with a melting point of that can be synthesized by partial hydrolysis of , or the reaction of with silica or : : + → + + A high-yield synthesi .... : XeO3 + XeOF4 -> 2XeO2F2 References {{Inorganic-stub Nonmetal halides Oxyfluorides Xenon(VI) compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tellurium Tetrafluoride
Tellurium tetrafluoride, TeF4, is a stable, white, hygroscopic crystalline solid and is one of two fluorides of tellurium. The other binary fluoride is tellurium hexafluoride.''Inorganic Chemistry'',Egon Wiberg, Arnold Frederick Holleman Elsevier 2001 The widely reported Te2F10 has been shown to be F5TeOTeF5 There are other tellurium compounds that contain fluorine, but only the two mentioned contain solely tellurium and fluorine. Tellurium difluoride, TeF2, and ditellurium difluoride, Te2F2 are not known. Preparation Tellurium tetrafluoride can be prepared by the following reaction: : TeO2 + 2 SF4 → TeF4 + 2 SOF2 It is also prepared by reacting nitryl fluoride with tellurium or from the elements at 0 °C or by reacting selenium tetrafluoride with tellurium dioxide at 80 °C. Fluorine in nitrogen can react with TeCl2 or TeBr2 to form TeF4. PbF2 will also fluorinate tellurium to TeF4. Reactivity Tellurium tetrafluoride will react with water or silica and form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenium Tetrafluoride
Selenium tetrafluoride ( Se F4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas. Synthesis The first reported synthesis of selenium tetrafluoride was by Paul Lebeau in 1907, who treated selenium with fluorine: :Se + 2 F2 → SeF4 A synthesis involving more easily handled reagents entails the fluorination of selenium dioxide with sulfur tetrafluoride: :SF4 + SeO2 → SeF4 + SO2 An intermediate in this reaction is seleninyl fluoride (SeOF2). Other methods of preparation include fluorinating elemental selenium with chlorine trifluoride: :3 Se + 4 ClF3 → 3 SeF4 + 2 Cl2 Structure and bonding Selenium in SeF4 has an oxidation state of +4. Its shape in the gaseous phase is similar to that of SF4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berry Pseudorotation
The Berry mechanism, or Berry pseudorotation mechanism, is a type of vibration causing molecules of certain geometries to isomerize by exchanging the two axial ligands (see Figure at right) for two of the equatorial ones. It is the most widely accepted mechanism for pseudorotation and most commonly occurs in trigonal bipyramidal molecules such as PF5, though it can also occur in molecules with a square pyramidal geometry. The Berry mechanism is named after R. Stephen Berry, who first described this mechanism in 1960.RS Berry, 1960, "Correlation of rates of intramolecular tunneling processes, with application to some Group V compounds," ''J. Chem. Phys.'' 32:933-938, DOI 10.1063/1.1730820; seo accessed 28 May 2014M Cass, KK Hii & HS Rzepa, 2005, "Mechanisms that interchange axial and equatorial atoms in fluxional processes: Illustration of the Berry pseudorotation, the turnstile and the lever mechanisms via animation of transition state normal vibrational modes", ''J. Chem. Educ. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trigonal Bipyramidal Molecular Geometry
In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. This is one geometry for which the bond angles surrounding the central atom are not identical (see also pentagonal bipyramid), because there is no geometrical arrangement with five terminal atoms in equivalent positions. Examples of this molecular geometry are phosphorus pentafluoride (), and phosphorus pentachloride () in the gas phase. Axial (or apical) and equatorial positions The five atoms bonded to the central atom are not all equivalent, and two different types of position are defined. For phosphorus pentachloride as an example, the phosphorus atom shares a plane with three chlorine atoms at 120° angles to each other in ''equatorial'' positions, and two more chlorine atoms above and below the plane (''axial'' or ''apical'' positions). According to the VSEPR theory of molecular geometry, an axial position is more crowd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lone Pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone pairs are found in the outermost electron shell of atoms. They can be identified by using a Lewis structure. Electron pairs are therefore considered lone pairs if two electrons are paired but are not used in chemical bonding. Thus, the number of electrons in lone pairs plus the number of electrons in bonds equals the number of valence electrons around an atom. Lone pair is a concept used in valence shell electron pair repulsion theory (VSEPR theory) which explains the shapes of molecules. They are also referred to in the chemistry of Lewis acids and bases. However, not all non-bonding pairs of electrons are considered by chemists to be lone pairs. Examples are the transition metals where the non-bonding pairs do not influence molecular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Tetrafluoride
Sulfur tetrafluoride is the chemical compound with the formula S F4. It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Despite these unwelcome characteristics, this compound is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries. Structure Sulfur in SF4 is in the formal +4 oxidation state. Of sulfur's total of six valence electrons, two form a lone pair. The structure of SF4 can therefore be anticipated using the principles of VSEPR theory: it is a see-saw shape, with S at the center. One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. Consequently, the molecule has two distinct types of F ligands, two axial and two equatorial. The relevant bond distances are = 164.3 pm and = 154.2 pm. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly. In c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



4-3D-balls.png)