|
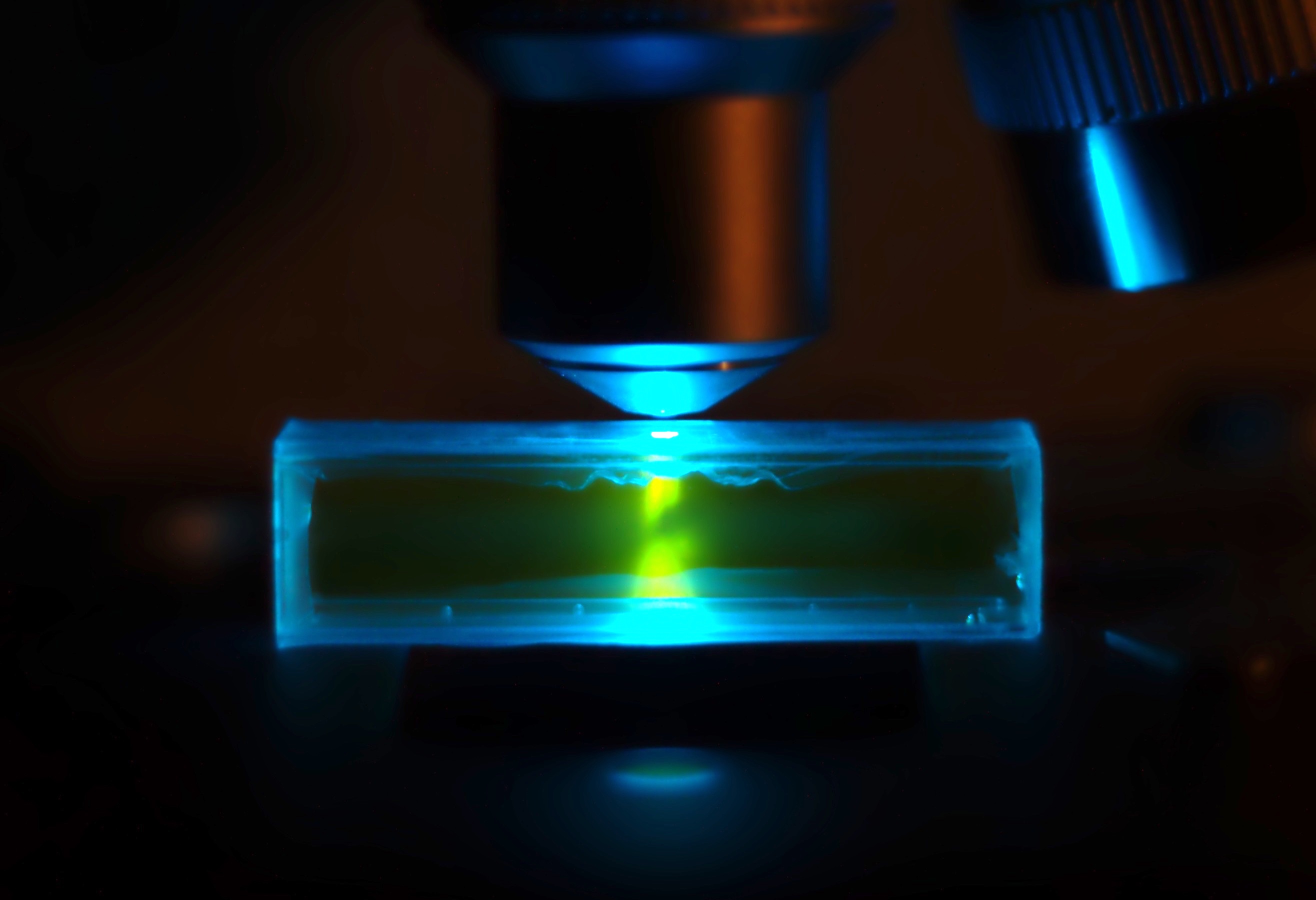
SYBR Green
SYBR Green I (SG) is an asymmetrical cyanine dye used as a nucleic acid stain in molecular biology. The SYBR family of dyes is produced by Molecular Probes Inc., now owned by Thermo Fisher Scientific. SYBR Green I binds to DNA. The resulting DNA-dye-complex best absorbs 497 nanometer blue light (λmax = 497 nm) and emits green light (λmax = 520 nm). The stain preferentially binds to double-stranded DNA, but will stain single-stranded (ss) DNA with lower performance. SYBR Green can also stain RNA with a lower performance than ssDNA. Uses SYBR Green finds usage in several areas of biochemistry and molecular biology. It is used as a dye for the quantification of double stranded DNA in some methods of quantitative PCR. It is also used to visualise DNA in gel electrophoresis. Higher concentrations of SYBR Green can be used to stain agarose gels in order to visualise the DNA present. In addition to labelling pure nucleic acids, SYBR Green can also be used for labelling o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence Microscopy
A fluorescence microscope is an optical microscope that uses fluorescence instead of, or in addition to, scattering, reflection, and attenuation or absorption, to study the properties of organic or inorganic substances. "Fluorescence microscope" refers to any microscope that uses fluorescence to generate an image, whether it is a simple set up like an epifluorescence microscope or a more complicated design such as a confocal microscope, which uses optical sectioning to get better resolution of the fluorescence image. Principle The specimen is illuminated with light of a specific wavelength (or wavelengths) which is absorbed by the fluorophores, causing them to emit light of longer wavelengths (i.e., of a different color than the absorbed light). The illumination light is separated from the much weaker emitted fluorescence through the use of a spectral emission filter. Typical components of a fluorescence microscope are a light source (xenon arc lamp or mercury-vapor lamp are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Staining Dyes
Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology (microscopic study of biological tissues), in cytology (microscopic study of cells), and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of diseases at the microscopic level. Stains may be used to define biological tissues (highlighting, for example, muscle fibers or connective tissue), cell populations (classifying different blood cells), or organelles within individual cells. In biochemistry, it involves adding a class-specific ( DNA, proteins, lipids, carbohydrates) dye to a substrate to qualify or quantify the presence of a specific compound. Staining and fluorescent tagging can serve similar purposes. Biological staining is also used to mark cells in flow cytometry, and to flag proteins or nucleic acids in gel electrophoresis. Light microscopes are used for viewing st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GelGreen
GelGreen is an intercalating nucleic acid stain used in molecular genetics for agarose gel DNA electrophoresis. GelGreen consists of two acridine orange subunits that are bridged by a linear oxygenated spacer. Its fluorophore, and therefore its optical properties, are essentially identical to those of other ''N''-alkylacridinium orange dyes. When exposed to ultraviolet light, it will fluoresce with a greenish color that strongly intensifies after binding to DNA. The substance is marketed as a less toxic and more sensitive alternative to ethidium bromide. GelGreen is sold as a solution in either DMSO or water. {{short description, DNA gel stain for molecular genetics See also * Ethidium bromide * GelRed * SYBR Green I * Agarose gel electrophoresis and gel electrophoresis of nucleic acids * Acridine orange Acridine orange is an organic compound that serves as a nucleic acid-selective fluorescent dye with cationic properties useful for cell cycle determination. Acridine oran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxazole Yellow
Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon. Oxazoles are aromatic compounds but less so than the thiazoles. Oxazole is a weak base; its conjugate acid has a p''K''a of 0.8, compared to 7 for imidazole. Preparation Classical oxazole synthetic methods in organic chemistry are * the Robinson–Gabriel synthesis by dehydration of 2-acylaminoketones * the Fischer oxazole synthesis from cyanohydrins and aldehydes * the Bredereck reaction with α-haloketones and formamide * the Van Leusen reaction with aldehydes and TosMIC Other methods: * Oxazolines can also be obtained from cycloisomerization of certain propargyl amides. In one study oxazoles were prepared via a one-pot synthesis consisting of the condensation of propargyl amine and benzoyl chloride to the amide, followed by a Sonogashira coupling of the terminal alkyne end with another equivalent of benzoylchlor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiazole Orange
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1). Molecular and electronic structure Thiazoles are members of the azoles, heterocycles that include imidazoles and oxazoles. Thiazole can also be considered a functional group. Oxazoles are related compounds, with sulfur replaced by oxygen. Thiazoles are structurally similar to imidazoles, with the thiazole sulfur replaced by nitrogen. Thiazole rings are planar and aromatic. Thiazoles are characterized by larger pi-electron delocalization than the corresponding oxazoles and have therefore greater aromaticity. This aromaticity is evidenced by the chemical shift of the ring protons in proton NMR spectroscopy (between 7.27 and 8.77 ppm), clearly indicating ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SYBR Gold
SYBR Gold is an asymmetrical cyanine dye. It can be used as a stain for double-stranded DNA, single-stranded DNA, and RNA. SYBR Gold is the most sensitive fluorescent stain of the SYBR family of dyes for the detection of nucleic acids. The SYBR family of dyes is produced by Molecular Probes Inc., now owned by Thermo Fisher Scientific SYBR Gold is more sensitive than ethidium bromide, SYBR Green I, and SYBR Green II for detecting various types of nucleic acids. SYBR Gold's superior sensitivity is due to the high fluorescence quantum yield of the dye-nucleic acid complexes (~0.6-0.7) and the dye's large fluorescence enhancement upon binding to nucleic acids (~1000-fold). SYBR Gold can detect as little as 25 pg of DNA which makes it >10-fold more sensitive than ethidium bromide for detecting nucleic acids in denaturing urea, gyoxal, and formaldehyde gels, even with 300 nm transillumination. Fluorescence properties SYBR Gold has two fluorescence excitation maxima when bound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SYBR Safe
SYBR Safe is a cyanine dye used as a nucleic acid stain in molecular biology. SYBR Safe is one of a number of SYBR dyes made by the Life Technologies Corporation. SYBR Safe binds to DNA. The resulting DNA-dye-complex absorbs blue light (λmax = 509 nm) and emits green light (λmax = 524 nm). Safety SYBR Safe is marketed as a safer alternative to ethidium bromide. However, as the molecule itself is quite a bit larger than ethidium bromide, it does not bind to the column of a gel extraction as easily, making it less efficient when trying to clone a DNA fragment into a plasmid. SYBR Safe has a very similar structure to thiazole orange, which has a methyl group attached to the charged nitrogen, whereas SYBR Safe has an ''N''-propyl group. Thiazole Orange has been shown to be three to four times less mutagenic than ethidium bromide whereas SYBR Safe is four to five times less mutagenic. Additionally, according to the Life Technologies website, SYBR Safe is not lethal in ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carcinogen
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive substances are considered carcinogens, but their carcinogenic activity is attributed to the radiation, for example gamma rays and alpha particles, which they emit. Common examples of non-radioactive carcinogens are inhaled asbestos, certain dioxins, and tobacco smoke. Although the public generally associates carcinogenicity with synthetic chemicals, it is equally likely to arise from both natural and synthetic substances. Carcinogens are not necessarily immediately toxic; thus, their effect can be insidious. Carcinogens, as mentioned, are agents in the environment capable of contributing to cancer growth. Carcinogens can be categorized into two different types: activation-dependent and activation-independent, and each nature impacts their level ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Small Molecule
Within the fields of molecular biology and pharmacology, a small molecule or micromolecule is a low molecular weight (≤ 1000 daltons) organic compound that may regulate a biological process, with a size on the order of 1 nm. Many drugs are small molecules; the terms are equivalent in the literature. Larger structures such as nucleic acids and proteins, and many polysaccharides are not small molecules, although their constituent monomers (ribo- or deoxyribonucleotides, amino acids, and monosaccharides, respectively) are often considered small molecules. Small molecules may be used as research tools to probe biological function as well as leads in the development of new therapeutic agents. Some can inhibit a specific function of a protein or disrupt protein–protein interactions. Pharmacology usually restricts the term "small molecule" to molecules that bind specific biological macromolecules and act as an effector, altering the activity or function of the target. Small ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |