|
SEM-EDX
Energy-dispersive X-ray spectroscopy (EDS, EDX, EDXS or XEDS), sometimes called energy dispersive X-ray analysis (EDXA or EDAX) or energy dispersive X-ray microanalysis (EDXMA), is an analytical technique used for the elemental analysis or chemical characterization of a sample. It relies on an interaction of some source of X-ray excitation and a sample. Its characterization capabilities are due in large part to the fundamental principle that each element has a unique atomic structure allowing a unique set of peaks on its electromagnetic emission spectrum (which is the main principle of spectroscopy). The peak positions are predicted by the Moseley's law with accuracy much better than experimental resolution of a typical EDX instrument. To stimulate the emission of characteristic X-rays from a specimen a beam of electrons is focused into the sample being studied. At rest, an atom within the sample contains ground state (or unexcited) electrons in discrete energy levels or elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Fluorescence
X-ray fluorescence (XRF) is the emission of characteristic "secondary" (or fluorescent) X-rays from a material that has been excited by being bombarded with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and analytical chemistry, chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science, archaeology and art objects such as paintings. Underlying physics When materials are exposed to short-wavelength X-rays or to gamma rays, ionization of their component atoms may take place. Ionization consists of the ejection of one or more electrons from the atom, and may occur if the atom is exposed to radiation with an energy greater than its ionization energy. X-rays and gamma rays can be energetic enough to expel tightly held electrons from the inner atomic orbital, orbitals of the atom. The removal of an electron in this way makes the electronic structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microphonic
Microphonics, microphony, or microphonism describes the phenomenon wherein certain components in electronic devices transform mechanical vibrations into an undesired electrical signal (noise). The term comes from analogy with a microphone, which is intentionally designed to convert vibrations to electrical signals. Description When electronic equipment was built using vacuum tubes, microphonics were often a serious design problem. The charged elements in the vacuum tubes can mechanically vibrate, changing the distance between the elements, producing charge flows in and out of the tube in a manner identical to a capacitor microphone. A system sufficiently susceptible to microphonics could experience audio feedback, and make noises if jarred or bumped. To minimize these effects, some vacuum tubes were made with thicker internal insulating plates and more supports, and tube-socket assemblies were sometimes shock-mounted by means of small rubber grommets placed in the screw holes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diffraction
Diffraction is defined as the interference or bending of waves around the corners of an obstacle or through an aperture into the region of geometrical shadow of the obstacle/aperture. The diffracting object or aperture effectively becomes a secondary source of the propagating wave. Italian scientist Francesco Maria Grimaldi coined the word ''diffraction'' and was the first to record accurate observations of the phenomenon in 1660. In classical physics, the diffraction phenomenon is described by the Huygens–Fresnel principle that treats each point in a propagating wavefront as a collection of individual spherical wavelets. The characteristic bending pattern is most pronounced when a wave from a coherent source (such as a laser) encounters a slit/aperture that is comparable in size to its wavelength, as shown in the inserted image. This is due to the addition, or interference, of different points on the wavefront (or, equivalently, each wavelet) that travel by paths of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wavelength Dispersive X-ray Spectroscopy
Wavelength-dispersive X-ray spectroscopy (WDXS or WDS) is a non-destructive analysis technique used to obtain elemental information about a range of materials by measuring characteristic x-rays within a small wavelength range. The technique generates a spectrum in which the peaks correspond to specific x-ray lines and elements can be easily identified. WDS is primarily used in chemical analysis, wavelength dispersive X-ray fluorescence (WDXRF) spectrometry, electron microprobes, scanning electron microscopes, and high precision experiments for testing atomic and plasma physics. Theory Wavelength-dispersive X-ray spectroscopy is based on known principles of how the characteristic x-rays are generated by a sample and how the x-rays are measured. X-ray generation X-rays are generated when an electron beam of high enough energy dislodges an electron from an inner orbital within an atom or ion, creating a void. This void is filled when an electron from a higher orbital releases ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding Energy
In physics and chemistry, binding energy is the smallest amount of energy required to remove a particle from a system of particles or to disassemble a system of particles into individual parts. In the former meaning the term is predominantly used in condensed matter physics, atomic physics, and chemistry, whereas in nuclear physics the term '' separation energy'' is used. A bound system is typically at a lower energy level than its unbound constituents. According to relativity theory, a decrease in the total energy of a system is accompanied by a decrease in the total mass, where . Types of binding energy There are several types of binding energy, each operating over a different distance and energy scale. The smaller the size of a bound system, the higher its associated binding energy. Mass–energy relation A bound system is typically at a lower energy level than its unbound constituents because its mass must be less than the total mass of its unbound constituents. For s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinetic Energy
In physics, the kinetic energy of an object is the energy that it possesses due to its motion. It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes. The same amount of work is done by the body when decelerating from its current speed to a state of rest. Formally, a kinetic energy is any term in a system's Lagrangian which includes a derivative with respect to time. In classical mechanics, the kinetic energy of a non-rotating object of mass ''m'' traveling at a speed ''v'' is \fracmv^2. In relativistic mechanics, this is a good approximation only when ''v'' is much less than the speed of light. The standard unit of kinetic energy is the joule, while the English unit of kinetic energy is the foot-pound. History and etymology The adjective ''kinetic'' has its roots in the Greek word κίνησι� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
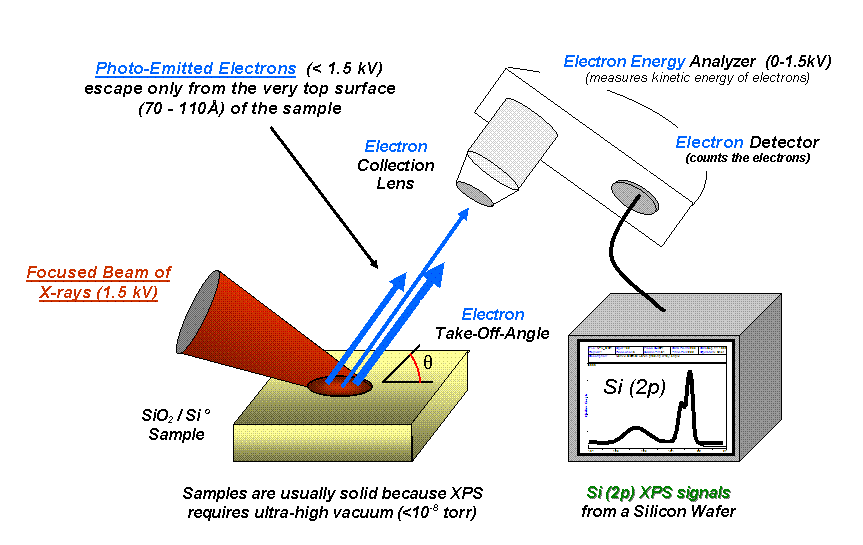
X-ray Photoelectron Spectroscopy
X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique based on the photoelectric effect that can identify the elements that exist within a material (elemental composition) or are covering its surface, as well as their chemical state, and the overall electronic structure and density of the electronic states in the material. XPS is a powerful measurement technique because it not only shows what elements are present, but also what other elements they are bonded to. The technique can be used in line profiling of the elemental composition across the surface, or in depth profiling when paired with ion-beam etching. It is often applied to study chemical processes in the materials in their as-received state or after cleavage, scraping, exposure to heat, reactive gasses or solutions, ultraviolet light, or during ion implantation. XPS belongs to the family of photoemission spectroscopies in which electron population spectra are obtained by irr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Auger Electron Spectroscopy
A Hanford scientist uses an Auger electron spectrometer to determine the elemental composition of surfaces. Auger electron spectroscopy (AES; pronounced in French) is a common analytical technique used specifically in the study of surfaces and, more generally, in the area of materials science. It is a form of electron spectroscopy that relies on the Auger effect, based on the analysis of energetic electrons emitted from an excited atom after a series of internal relaxation events. The Auger effect was discovered independently by both Lise Meitner and Pierre Auger in the 1920s. Though the discovery was made by Meitner and initially reported in the journal ''Zeitschrift für Physik'' in 1922, Auger is credited with the discovery in most of the scientific community. Until the early 1950s Auger transitions were considered nuisance effects by spectroscopists, not containing much relevant material information, but studied so as to explain anomalies in X-ray spectroscopy data. Since 19 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Auger Electron
The Auger effect or Auger−Meitner effect is a physical phenomenon in which the filling of an inner-shell vacancy of an atom is accompanied by the emission of an electron from the same atom. When a core electron is removed, leaving a vacancy, an electron from a higher energy level may fall into the vacancy, resulting in a release of energy. Although most often this energy is released in the form of an emitted photon, the energy can also be transferred to another electron, which is ejected from the atom; this second ejected electron is called an Auger electron. Effect The effect was first discovered by Lise Meitner in 1922; Pierre Victor Auger independently discovered the effect shortly after and is credited with the discovery in most of the scientific community. Upon ejection, the kinetic energy of the Auger electron corresponds to the difference between the energy of the initial electronic transition into the vacancy and the ionization energy for the electron shell from which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermoelectric Cooling
Thermoelectric cooling uses the Peltier effect to create a heat flux at the junction of two different types of materials. A Peltier cooler, heater, or thermoelectric heat pump is a solid-state active heat pump which transfers heat from one side of the device to the other, with consumption of electrical energy, depending on the direction of the current. Such an instrument is also called a Peltier device, Peltier heat pump, solid state refrigerator, or thermoelectric cooler (TEC) and occasionally a thermoelectric battery. It can be used either for heating or for cooling, although in practice the main application is cooling. It can also be used as a temperature controller that either heats or cools. This technology is far less commonly applied to refrigeration than vapor-compression refrigeration is. The primary advantages of a Peltier cooler compared to a vapor-compression refrigerator are its lack of moving parts or circulating liquid, very long life, invulnerability to leaks, s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |