|
SAP1a
SAP1A is one of a family of proteins that contains a unique DNA binding domain termed the ETS domain. Transcription The transcriptional activation domain of SAP1a resides within the C-terminal region, the function of which may be impeded by the N-terminus. Several potential Extracellular signal-regulated kinases, ERK consensus sites within the C-terminal region of SAP1a can modulate its transactivation efficacy, implicating that SAP1a is a direct target of ERKs. Interactions SAP1a has been shown to interact with the c-fos serum response element upon recruitment by the serum response factor. SAP1a is a nuclear protein stimulating transcription (genetics), transcription via the c-fos serum response element, and additionally via an Ets binding site independently of the serum response factor. Insulin activated the human INSIG2 promoter in a process mediated by phosphorylated SAP1a. Sap1a is phosphorylated efficiently by ERKs but not by SAPK/JNKs. Serum response factor-dependent tern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FLI1
Friend leukemia integration 1 transcription factor (FLI1), also known as transcription factor ERGB, is a protein that in humans is encoded by the ''FLI1'' gene, which is a proto-oncogene. Function Fli-1 is a member of the ETS transcription factor family that was first identified in erythroleukemias induced by Friend Murine Leukemia Virus ( F-MuLV). Fli-1 is activated through retroviral insertional mutagenesis in 90% of F-MuLV-induced erythroleukemias. The constitutive activation of fli-1 in erythroblasts leads to a dramatic shift in the Epo/ Epo-R signal transduction pathway, blocking erythroid differentiation, activating the Ras pathway, and resulting in massive Epo-independent proliferation of erythroblasts. These results suggest that Fli-1 overexpression in erythroblasts alters their responsiveness to Epo and triggers abnormal proliferation by switching the signaling event(s) associated with terminal differentiation to proliferation. Clinical significance In addition ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid resid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
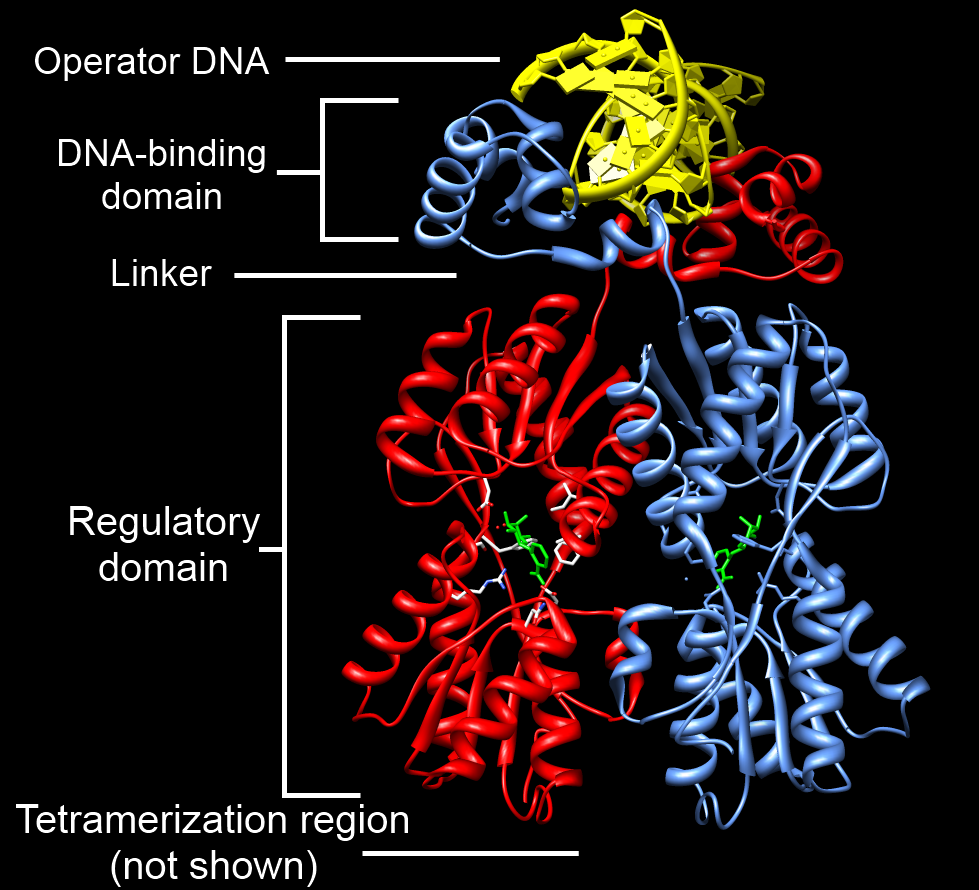
DNA Binding Domain
A DNA-binding domain (DBD) is an independently folded protein domain that contains at least one structural motif that recognizes double- or single-stranded DNA. A DBD can recognize a specific DNA sequence (a recognition sequence) or have a general affinity to DNA. Some DNA-binding domains may also include nucleic acids in their folded structure. Function One or more DNA-binding domains are often part of a larger protein consisting of further protein domains with differing function. The extra domains often regulate the activity of the DNA-binding domain. The function of DNA binding is either structural or involves transcription regulation, with the two roles sometimes overlapping. DNA-binding domains with functions involving DNA structure have biological roles in DNA replication, repair, storage, and modification, such as methylation. Many proteins involved in the regulation of gene expression contain DNA-binding domains. For example, proteins that regulate transcription by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extracellular Signal-regulated Kinases
In molecular biology, extracellular signal-regulated kinases (ERKs) or classical MAP kinases are widely expressed protein kinase intracellular signalling molecules that are involved in functions including the regulation of meiosis, mitosis, and postmitotic functions in differentiated cells. Many different stimuli, including growth factors, cytokines, virus infection, ligands for heterotrimeric G protein-coupled receptors, transforming agents, and carcinogens, activate the ERK pathway. The term, "extracellular signal-regulated kinases", is sometimes used as a synonym for mitogen-activated protein kinase (MAPK), but has more recently been adopted for a specific subset of the mammalian MAPK family. In the MAPK/ERK pathway, Ras activates c-Raf, followed by mitogen-activated protein kinase kinase (abbreviated as MKK, MEK, or MAP2K) and then MAPK1/2 (below). Ras is typically activated by growth hormones through receptor tyrosine kinases and GRB2/SOS, but may also receive o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-fos
Protein c-Fos is a proto-oncogene that is the human homolog of the retroviral oncogene v-fos. It is encoded in humans by the ''FOS'' gene. It was first discovered in rat fibroblasts as the transforming gene of the FBJ MSV (Finkel–Biskis–Jinkins murine osteogenic sarcoma virus) (Curran and Tech, 1982). It is a part of a bigger Fos family of transcription factors which includes c-Fos, FosB, Fra-1 and Fra-2. It has been mapped to chromosome region 14q21→q31. c-Fos encodes a 62 kDa protein, which forms heterodimer with c-jun (part of Jun family of transcription factors), resulting in the formation of AP-1 (Activator Protein-1) complex which binds DNA at AP-1 specific sites at the promoter and enhancer regions of target genes and converts extracellular signals into changes of gene expression. It plays an important role in many cellular functions and has been found to be overexpressed in a variety of cancers. Structure and function c-Fos is a 380 amino acid protein with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transcription (genetics)
Transcription is the process of copying a segment of DNA into RNA. The segments of DNA transcribed into RNA molecules that can encode proteins are said to produce messenger RNA (mRNA). Other segments of DNA are copied into RNA molecules called non-coding RNAs (ncRNAs). mRNA comprises only 1–3% of total RNA samples. Less than 2% of the human genome can be transcribed into mRNA ( Human genome#Coding vs. noncoding DNA), while at least 80% of mammalian genomic DNA can be actively transcribed (in one or more types of cells), with the majority of this 80% considered to be ncRNA. Both DNA and RNA are nucleic acids, which use base pairs of nucleotides as a complementary language. During transcription, a DNA sequence is read by an RNA polymerase, which produces a complementary, antiparallel RNA strand called a primary transcript. Transcription proceeds in the following general steps: # RNA polymerase, together with one or more general transcription factors, binds to promot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serum Response Factor
Serum response factor, also known as SRF, is a transcription factor protein. Function Serum response factor is a member of the MADS (MCM1, Agamous, Deficiens, and SRF) box superfamily of transcription factors. This protein binds to the serum response element (SRE) in the promoter region of target genes. This protein regulates the activity of many immediate early genes, for example c-fos, and thereby participates in cell cycle regulation, apoptosis, cell growth, and cell differentiation. This gene is the downstream target of many pathways; for example, the mitogen-activated protein kinase pathway (MAPK) that acts through the ternary complex factors (TCFs). SRF is important during the development of the embryo, as it has been linked to the formation of mesoderm. In the fully developed mammal, SRF is crucial for the growth of skeletal muscle. Interaction of SRF with other proteins, such as steroid hormone receptors, may contribute to regulation of muscle growth by steroids. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EGR1
EGR-1 (Early growth response protein 1) also known as ZNF268 (zinc finger protein 268) or NGFI-A (nerve growth factor-induced protein A) is a protein that in humans is encoded by the ''EGR1'' gene. EGR-1 is a mammalian transcription factor. It was also named Krox-24, TIS8, and ZENK. It was originally discovered in mice. Function The protein encoded by this gene belongs to the EGR family of Cys2His2-type zinc finger proteins. It is a nuclear protein and functions as a transcriptional regulator. The products of target genes it activates are required for differentiation and mitogenesis. Studies suggest this is a tumor suppressor gene. It has a distinct pattern of expression in the brain, and its induction has been shown to be associated with neuronal activity. Several studies suggest it has a role in neuronal plasticity. EGR-1 is an important transcription factor in memory formation. It has an essential role in brain neuron epigenetic reprogramming. EGR-1 recruits the TET1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |