|
Rosenmund Reduction
The Rosenmund reduction is a hydrogenation process in which an acyl chloride is selectively reduced to an aldehyde. The reaction was named after Karl Wilhelm Rosenmund, who first reported it in 1918. The reaction, a hydrogenolysis, is catalysed by palladium on barium sulfate, which is sometimes called the Rosenmund catalyst. Barium sulfate has a low surface area which reduces the activity of the palladium, preventing over-reduction. However, for certain reactive acyl chlorides the activity must be reduced further, by the addition of a poison. Originally this was thioquinanthrene although thiourea has also been used. Deactivation is required because the system must reduce the acyl chloride but not the subsequent aldehyde. If further reduction does take place it will create a primary alcohol which would then react with the remaining acyl chloride to form an ester. Rosenmund catalyst can be prepared by reduction of palladium(II) chloride solution in the presence of BaSO4. Typical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Karl Wilhelm Rosenmund
Karl Wilhelm Louis Rosenmund (15 December 1884 – 8 February 1965) was a German chemist. He was born in Berlin and died in Kiel. Rosenmund studied chemistry and received his Ph.D. 1906 from University of Berlin for his work with Otto Diels. He discovered the Rosenmund reduction, which is the reduction of acid chlorides to aldehydes over palladium on barium sulfate as catalyst (Lindlar catalyst). The Rosenmund–von Braun reaction, the conversion of an aryl bromide to an aryl nitrile is also named after him. Rosenmund-Kuhnhenn method is suitable for the determination of iodine value in conjugated systems (ASTM ASTM International, formerly known as American Society for Testing and Materials, is an international standards organization that develops and publishes voluntary consensus technical standards for a wide range of materials, products, systems, an ... D1541). References * 1884 births 1965 deaths 20th-century German chemists Scientists from Berlin {{Germ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalyst Poisoning
Catalyst poisoning refers to the partial or total deactivation of a catalyst by a chemical compound. Poisoning refers specifically to chemical deactivation, rather than other mechanisms of catalyst degradation such as thermal decomposition or physical damage. Although usually undesirable, poisoning may be helpful when it results in improved catalyst selectivity (e.g. Lindlar's catalyst). An important historic example was the poisoning of catalytic converters by leaded fuel. Poisoning of Pd catalysts Organic functional groups and inorganic anions often have the ability to strongly adsorb to metal surfaces. Common catalyst poisons include carbon monoxide, halides, cyanides, sulfides, sulfites, phosphates, phosphites and organic molecules such as nitriles, nitro compounds, oximes, and nitrogen-containing heterocycles. Agents vary their catalytic properties because of the nature of the transition metal. Lindlar catalysts are prepared by the reduction of palladium chloride in a slurry o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stephen Aldehyde Synthesis
Stephen aldehyde synthesis, a named reaction in chemistry, was invented by Henry Stephen ( OBE/MBE). This reaction involves the preparation of aldehydes (R-CHO) from nitriles (R-CN) using tin(II) chloride (SnCl2), hydrochloric acid (HCl) and quenching the resulting iminium salt ( -CH=NH2sup>+Cl−) with water (H2O). During the synthesis, ammonium chloride is also produced. Mechanism The following scheme shows the reaction mechanism: By addition of hydrogen chloride the used nitrile (1) reacts to its corresponding salt (2). It is believed that this salt is reduced by a single electron transfer by the tin(II) chloride (3a and 3b). The resulting salt (4) precipitates after some time as aldimine tin chloride (5). Hydrolysis of 5 produces a hemiaminal (6) from which an aldehyde (7) is formed. Substitutes that increase the electron density promote the formation of the aldimine-tin chloride adduct. With electron withdrawing substituents, the formation of an amide chloride is faci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diisobutylaluminium Hydride
Diisobutylaluminium hydride (DIBALH, DIBAL, DIBAL-H or DIBAH) is a reducing agent with the formula (''i''-Bu2AlH)2, where ''i''-Bu represents isobutyl (-CH2CH(CH3)2). This organoaluminium compound is a reagent in organic synthesis. Properties Like most organoaluminum compounds, the compound's structure is most probably more than that suggested by its empirical formula. A variety of techniques, not including X-ray crystallography, suggest that the compound exists as a dimer and a trimer, consisting of tetrahedral aluminium centers sharing bridging hydride ligands. Hydrides are small and, for aluminium derivatives, are highly basic, thus they bridge in preference to the alkyl groups. DIBAL can be prepared by heating triisobutylaluminium (itself a dimer) to induce beta-hydride elimination: :(''i''-Bu3Al)2 → (''i''-Bu2AlH)2 + 2 (CH3)2C=CH2 Although DIBAL can be purchased commercially as a colorless liquid, it is more commonly purchased and dispensed as a solution in an organic sol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grundmann Aldehyde Synthesis
The Grundmann aldehyde synthesis is a chemical reaction that produces an aldehyde from an acyl halide.Grundmann, C. ''Ann.'' 1936, ''524'', 31. Because of the Rosenmund reduction and DIBAL-H accomplish similar transformations, this reaction sequence is not practiced much currently. References Name reactions Organic redox reactions {{reaction-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
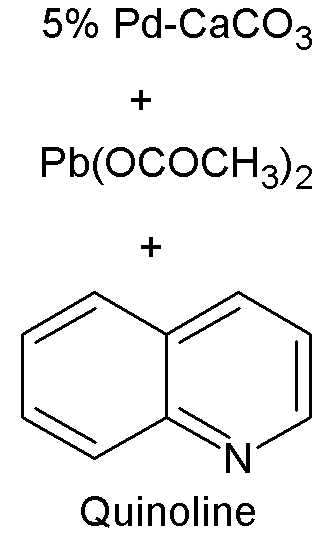
Lindlar Catalyst
A Lindlar catalyst is a heterogeneous catalyst that consists of palladium deposited on calcium carbonate or barium sulfate which is then poisoned with various forms of lead or sulfur. It is used for the hydrogenation of alkynes to alkenes (i.e. without further reduction into alkanes) and is named after its inventor Herbert Lindlar. Synthesis Lindlar catalyst is commercially available but may also be prepared by the reduction of palladium chloride in a slurry of calcium carbonate (CaCO3) followed by the addition of lead acetate. A variety of other "catalyst poisons" have been used, including lead oxide and quinoline. The palladium content of the supported catalyst is usually 5% by weight. Catalytic properties The catalyst is used for the hydrogenation of alkynes to alkenes (i.e. without further reduction into alkanes). The lead serves to deactivate the palladium sites, further deactivation of the catalyst with quinoline or 3,6-dithia-1,8-octanediol enhances its selectivity, pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature Etymology Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiourea
Thiourea () is an organosulfur compound with the formula and the structure . It is structurally similar to urea (), except that the oxygen atom is replaced by a sulfur atom (as implied by the ''thio-'' prefix); however, the properties of urea and thiourea differ significantly. Thiourea is a reagent in organic synthesis. "Thioureas" refer to a broad class of compounds with the general structure . Thioureas are related to thioamides, e.g. , where R is methyl, ethyl, etc. Structure and bonding Thiourea is a planar molecule. The C=S bond distance is 1.71 Å. The C-N distances average 1.33 Å. The weakening of the C-S bond by C-N pi-bonding is indicated by the short C=S bond in thiobenzophenone, which is 1.63 Å. Thiourea occurs in two tautomeric forms, of which the thione form predominates in aqueous solutions. The equilibrium constant has been calculated as ''K''eq is . The thiol form, which is also known as an isothiourea, can be encountered in substituted compounds such as i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioquinanthrene
Thioquinanthrene, also known as thiochinathren, is an aromatic organic chemical compound. It has the chemical formula C18H10N2S2 and reacts with alcoholates or alkoxides. One of the key uses is to act as a catalyst poison in the Rosenmund reduction. It has the IUPAC name of 2,13-dithia-10,21-diazapentacyclo 2.8.0.03,12.04,9.015,20ocosa-1(14),3(12),4,6,8,10,15,17,19,21-decaene. Rosenmund catalyst poison In the Rosenmeund reaction, an acid chloride is reduced to an aldehyde. Continuing the reduction produces an alcohol. This further reaction is undesirable as the alcohol will now react with the acyl chloride to produce the unwanted ester In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ... product. For this reaction (over reduction) to be prevented, the catalyst needs to be poisoned. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barium Sulfate
Barium sulfate (or sulphate) is the inorganic compound with the chemical formula Ba SO4. It is a white crystalline solid that is odorless and insoluble in water. It occurs as the mineral barite, which is the main commercial source of barium and materials prepared from it. The white opaque appearance and its high density are exploited in its main applications.Holleman, A. F. and Wiberg, E. (2001) ''Inorganic Chemistry'', San Diego, CA : Academic Press, . Uses Drilling fluids About 80% of the world's barium sulfate production, mostly purified mineral, is consumed as a component of oil well drilling fluid. It increases the density of the fluid, increasing the hydrostatic pressure in the well and reducing the chance of a blowout. Radiocontrast agent Barium sulfate in suspension is often used medically as a radiocontrast agent for X-ray imaging and other diagnostic procedures. It is most often used in imaging of the GI tract during what is colloquially known as a "barium meal". ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a Catalysis, catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated and unsaturated compounds, saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces Double bond, double and Triple bond, triple bonds in hydrocarbons. Process Hydrogenation has three components, the Saturated and unsaturated compounds, unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The redox, reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas. Palladium, platinum, rhodium, ruthenium, iridium and osmium form a group of elements referred to as the platinum group metals (PGMs). They have similar chemical properties, but palladium has the lowest melting point and is the least dense of them. More than half the supply of palladium and its congener platinum is used in catalytic converters, which convert as much as 90% of the harmful gases in automobile exhaust (hydrocarbons, carbon monoxide, and nitrogen dioxide) into nontoxic substances (nitrogen, carbon dioxide and water vapor). Palladium is also used in electronics, dentistry, medicine, hydrogen purification, chemical applications, groundwate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2.jpg)