|
Remdesivir
Remdesivir, sold under the brand name Veklury, Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged. is a broad-spectrum antiviral medication developed by the biopharmaceutical company Gilead Sciences. It is administered via injection into a vein. During the COVID‑19 pandemic, remdesivir was approved or authorized for emergency use to treat COVID‑19 in numerous countries. Remdesivir was originally developed to treat hepatitis C, and was subsequently investigated for Ebola virus disease and Marburg virus infections before being studied as a post-infection treatment for COVID‑19. Remdesivir is a prodrug that is intended to allow intracellular delivery of GS-441524 monophosphate and subsequent biotransformation into GS-441524 triphosphate, a ribonucleotide analogue inhibitor of viral RNA polymerase. The most common side effect in healthy volunteers is raised blood levels of liver enzymes. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GS-441524
GS-441524 is a nucleoside analogue antiviral drug which was developed by Gilead Sciences. It is the main plasma metabolite of the antiviral prodrug remdesivir, and has a half-life of around 24 hours in human patients. Remdesivir and GS-441524 were both found to be effective ''in vitro'' against feline coronavirus strains responsible for feline infectious peritonitis (FIP), a lethal systemic disease affecting domestic cats. Remdesivir was never tested in cats (though some vets now offer it), but GS-441524 has been found to be effective treatment for FIP and is widely used despite no official FDA approval due to Gilead's refusal to license this drug for veterinary use. An isopropylester pro-drug of GS-441524 - Obeldesivir has been developed by GILEAD_Sciences and is in Phase III clinical trials. A deuterated form has been developed by Vigonvita Life Sciences and is also in Phase III clinical trials. Use and research Since FIP is usually fatal and there are no approved treatmen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gilead Sciences
Gilead Sciences, Inc. () is an American biopharmaceutical company headquartered in Foster City, California, that focuses on researching and developing antiviral drugs used in the treatment of HIV/AIDS, hepatitis B, hepatitis C, influenza, and COVID-19, including ledipasvir/sofosbuvir and sofosbuvir. Gilead is a member of the NASDAQ Biotechnology Index and the S&P 500. Gilead was founded in 1987 under the name Oligogen by Michael L. Riordan. The original name was a reference to oligomers, small strands of DNA used to target genetic sequences. Gilead held its IPO in 1992, and successfully developed drugs like Tamiflu and Vistide that decade. In the 2000s, Gilead received approval for drugs including Viread and Hepsera, among others. It began evolving from a biotechnology company into a pharmaceutical company, acquiring several subsidiaries, though it still relied heavily on contracting to manufacture its drugs. The company continued its growth in the 2010s, but came under heavy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Baricitinib
Baricitinib, sold under the brand name Olumiant among others, is a medication used for the treatment of rheumatoid arthritis, alopecia areata, and COVID-19. It acts as an inhibitor of janus kinase (JAK), blocking the subtypes JAK1 and JAK2. Baricitinib is approved for medical use in the European Union Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged. and in the United States. An important side effect of JAK inhibitors is serious bacterial, mycobacterial, fungal and viral infections. Medical uses In February 2017, baricitinib was approved for use in the European Union as a second-line therapy for moderate to severe active rheumatoid arthritis in adults, either alone or in combination with methotrexate. In May 2018, the US Food and Drug Administration (FDA) approved baricitinib for the treatment of adults with moderately to severely active rheumatoid arthritis who have had an inadequate re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stat (website)
Stat (stylized STAT, sometimes also called Stat News) is an American health-oriented news website launched on November 4, 2015, by John W. Henry, the owner of ''The Boston Globe''. It is produced by Boston Globe Media and is headquartered in the ''Globe''s own building in Boston. Its executive editor is Rick Berke, who formerly worked at both ''The New York Times'' and ''Politico''. According to Kelsey Sutton of ''Politico'', the website is Henry's "biggest and most ambitious standalone site yet". The site's name comes from the term "stat", short for '' statim'', or "immediately"—a term that has long been used in medical contexts. As of February 2016, it had 45 staff members. Impact Notable stories Stat has broken include one about Robert Califf's research, published after then–President of the United States Barack Obama announced he would be his nominee to lead the Food and Drug Administration. The site also uncovered claims made by a vitamin company to which President Donal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Therapeutic Goods Administration
The Therapeutic Goods Administration (TGA) is the medicine and therapeutic regulatory agency of the Australian Government. As part of the Department of Health and Aged Care, the TGA regulates the quality, supply and advertising of medicines, pathology devices, medical devices, blood products and most other therapeutics. Any items that claim to have a therapeutic effect, are involved in the administration of medication, or are otherwise covered by the ''Therapeutic Goods Act 1989'', the ''Therapeutic Goods Regulations 1990'', or a ministerial order, must be approved by the TGA and registered in the Australian Register of Therapeutic Goods. Structure of the TGA and medical regulation in Australia In Australia, medical products are regulated by the TGA and, for controlled drugs such as cannabis, the Office of Drug Control (ODC). Together, the TGA and ODC form the Health Products Regulation Group within the Department of Health and Aged Care. The Health Products Regulation Group ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
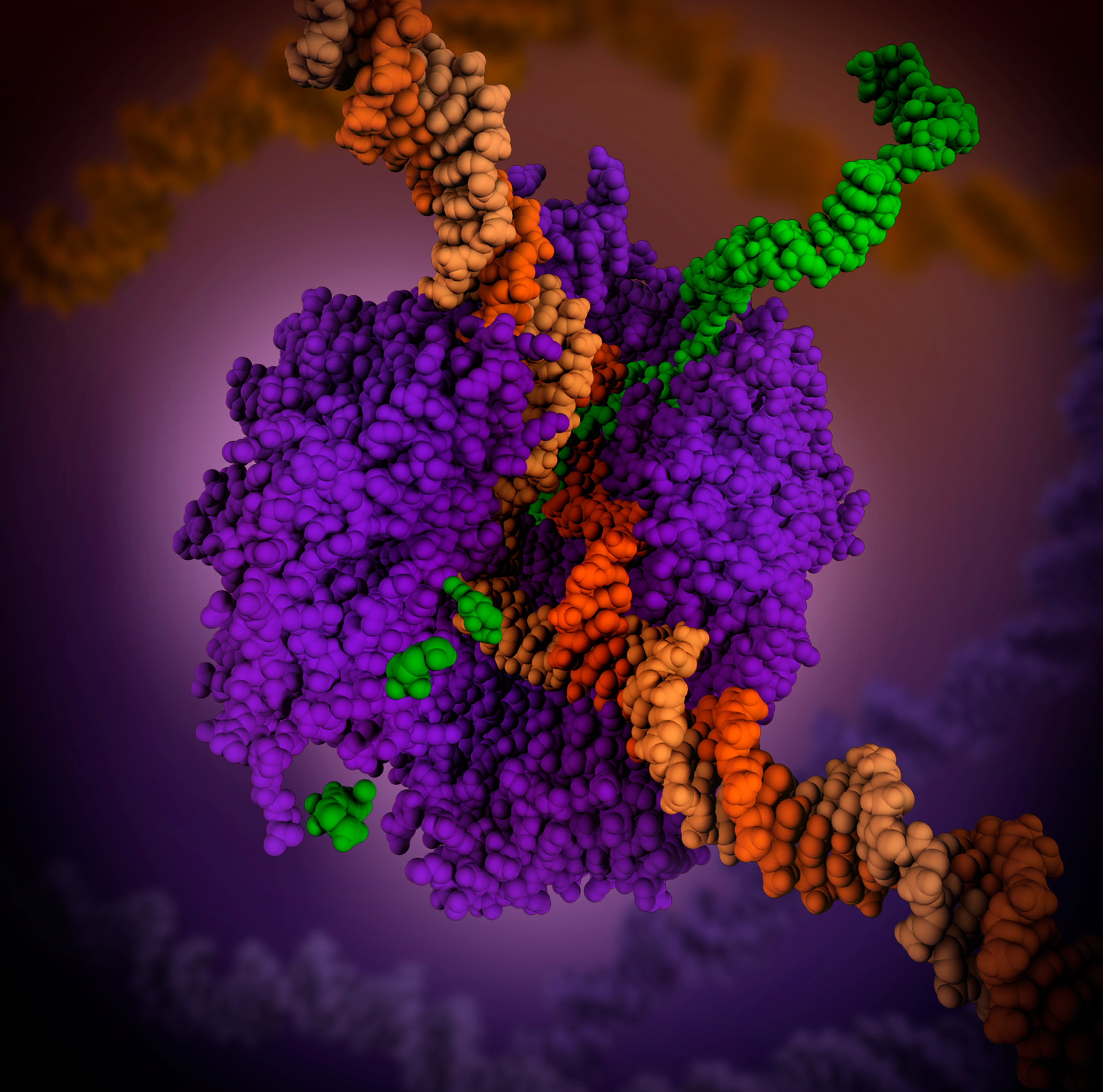
RNA Polymerase
In molecular biology, RNA polymerase (abbreviated RNAP or RNApol), or more specifically DNA-directed/dependent RNA polymerase (DdRP), is an enzyme that synthesizes RNA from a DNA template. Using the enzyme helicase, RNAP locally opens the double-stranded DNA so that one strand of the exposed nucleotides can be used as a template for the synthesis of RNA, a process called transcription. A transcription factor and its associated transcription mediator complex must be attached to a DNA binding site called a promoter region before RNAP can initiate the DNA unwinding at that position. RNAP not only initiates RNA transcription, it also guides the nucleotides into position, facilitates attachment and elongation, has intrinsic proofreading and replacement capabilities, and termination recognition capability. In eukaryotes, RNAP can build chains as long as 2.4 million nucleotides. RNAP produces RNA that, functionally, is either for protein coding, i.e. messenger RNA (mRNA); or n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomarker
In biomedical contexts, a biomarker, or biological marker, is a measurable indicator of some biological state or condition. Biomarkers are often measured and evaluated using blood, urine, or soft tissues to examine normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention. as cited in Biomarkers are used in many scientific fields. Medicine Biomarkers used in the medical field, are a part of a relatively new clinical toolset categorized by their clinical applications. The three main classes are molecular biomarkers, cellular biomarkers or imaging biomarkers. All three types of biomarkers have a clinical role in narrowing or guiding treatment decisions and follow a sub-categorization of being either predictive, prognostic, or diagnostic. Predictive Predictive molecular, cellular, or imaging biomarkers that pass validation can serve as a method of predicting clinical outcomes. Predictive biomarkers are used to help optimize id ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Respiratory Failure
Respiratory failure results from inadequate gas exchange by the respiratory system, meaning that the arterial oxygen, carbon dioxide, or both cannot be kept at normal levels. A drop in the oxygen carried in the blood is known as hypoxemia; a rise in arterial carbon dioxide levels is called hypercapnia. Respiratory failure is classified as either Type 1 or Type 2, based on whether there is a high carbon dioxide level, and can be acute or chronic. In clinical trials, the definition of respiratory failure usually includes increased respiratory rate, abnormal blood gases (hypoxemia, hypercapnia, or both), and evidence of increased work of breathing. Respiratory failure causes an altered mental status due to ischemia in the brain. The typical partial pressure reference values are oxygen Pa more than 80 mmHg (11 kPa) and carbon dioxide Pa less than 45 mmHg (6.0 kPa). Cause Several types of conditions can potentially result in respiratory failure: * Conditions that reduce the fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extracorporeal Membrane Oxygenation
Extracorporeal membrane oxygenation (ECMO), also known as extracorporeal life support (ECLS), is an extracorporeal technique of providing prolonged cardiac and respiratory support to persons whose heart and lungs are unable to provide an adequate amount of gas exchange or perfusion to sustain life. The technology for ECMO is largely derived from cardiopulmonary bypass, which provides shorter-term support with arrested native circulation. The device used is a membrane oxygenator, also known as an artificial lung. ECMO works by temporarily drawing blood from the body to allow artificial oxygenation of the red blood cells and removal of carbon dioxide. Generally, it is used either post-cardiopulmonary bypass or in late-stage treatment of a person with profound heart and/or lung failure, although it is now seeing use as a treatment for cardiac arrest in certain centers, allowing treatment of the underlying cause of arrest while circulation and oxygenation are supported. ECMO is als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pneumonia
Pneumonia is an inflammatory condition of the lung primarily affecting the small air sacs known as alveoli. Symptoms typically include some combination of productive or dry cough, chest pain, fever, and difficulty breathing. The severity of the condition is variable. Pneumonia is usually caused by infection with viruses or bacteria, and less commonly by other microorganisms. Identifying the responsible pathogen can be difficult. Diagnosis is often based on symptoms and physical examination. Chest X-rays, blood tests, and culture of the sputum may help confirm the diagnosis. The disease may be classified by where it was acquired, such as community- or hospital-acquired or healthcare-associated pneumonia. Risk factors for pneumonia include cystic fibrosis, chronic obstructive pulmonary disease (COPD), sickle cell disease, asthma, diabetes, heart failure, a history of smoking, a poor ability to cough (such as following a stroke), and a weak immune system. Vaccines to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indication (medicine)
In medicine, an indication is a valid reason to use a certain test, medication, procedure, or surgery. There can be multiple indications to use a procedure or medication. An indication can commonly be confused with the term diagnosis. A diagnosis is the assessment that a particular edicalcondition is present while an indication is a reason for use. The opposite of an indication is a contraindication, a reason to withhold a certain medical treatment because the risks of treatment clearly outweigh the benefits. In the United States, indications for prescription drugs are approved by the FDA. Indications are included in the Indications and Usage section of the Prescribing Information. The primary role of this section of labeling is to enable health care practitioners to readily identify appropriate therapies for patients by clearly communicating the drug’s approved indication(s). The Indications and Usage section states the disease or condition, or manifestation or symptoms thereof ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
First-in-class Medication
A first-in-class medication is a pharmaceutical that uses a "new and unique mechanism of action" to treat a particular medical condition. While the Food and Drug Administration's Center for Drug Evaluation and Research tracks first-in-class medications and reports on them annually, first-in-class is not considered a regulatory category. Although many first-in-class medications qualify as breakthrough therapies, Regenerative Medicine Advanced Therapies and/or orphan drugs, first-in-class status itself has no regulatory effect. Examples Controversy Safety By definition, a first-in-class drug does not have the safety evidence from analogous products that not-first-in-class drugs would have. However, a study investigating recalls and warnings in relation to first-in-class drugs approved between 1997 and 2012 by Health Canada has found that first-in-class drugs actually have a more favourable benefit-to-harm ratio. Economics First-in-class drugs are often seen as commerci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_I_(cropped).jpg)
_ECMO_for_cardiac_or_respiratory_failure.jpg)