|
Radioiodine
There are 37 known isotopes of iodine (53I) from 108I to 144I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element. Its longest-lived radioactive isotope, 129I, has a half-life of 15.7 million years, which is far too short for it to exist as a primordial nuclide. Cosmogenic sources of 129I produce very tiny quantities of it that are too small to affect atomic weight measurements; iodine is thus also a mononuclidic element—one that is found in nature only as a single nuclide. Most 129I derived radioactivity on Earth is man-made, an unwanted long-lived byproduct of early nuclear tests and nuclear fission accidents. All other iodine radioisotopes have half-lives less than 60 days, and four of these are used as tracers and therapeutic agents in medicine. These are 123I, 124I, 125I, and 131I. All industrial production of radioactive iodine isotopes involves these four useful radionuclides. The isotope 135I has a half-life less than s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine-131
Iodine-131 (131I, I-131) is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nuclear energy, medical diagnostic and treatment procedures, and natural gas production. It also plays a major role as a radioactive isotope present in nuclear fission products, and was a significant contributor to the health hazards from open-air atomic bomb testing in the 1950s, and from the Chernobyl disaster, as well as being a large fraction of the contamination hazard in the first weeks in the Fukushima nuclear crisis. This is because 131I is a major fission product of uranium and plutonium, comprising nearly 3% of the total products of fission (by weight). See fission product yield for a comparison with other radioactive fission products. 131I is also a major fission product of uranium-233, produced from thorium. Due to its mode of be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
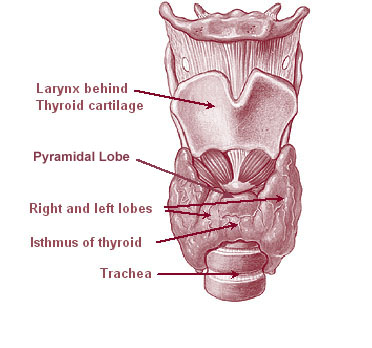
Thyroid
The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans it is in the neck and consists of two connected lobes. The lower two thirds of the lobes are connected by a thin band of tissue called the thyroid isthmus. The thyroid is located at the front of the neck, below the Adam's apple. Microscopically, the functional unit of the thyroid gland is the spherical thyroid follicle, lined with follicular cells (thyrocytes), and occasional parafollicular cells that surround a lumen containing colloid. The thyroid gland secretes three hormones: the two thyroid hormones triiodothyronine (T3) and thyroxine (T4)and a peptide hormone, calcitonin. The thyroid hormones influence the metabolic rate and protein synthesis, and in children, growth and development. Calcitonin plays a role in calcium homeostasis. Secretion of the two thyroid hormones is regulated by thyroid-stimulating hormone (TSH), which is secreted from the anterior pituitary gland. TSH is regula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fission Product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release of heat energy (kinetic energy of the nuclei), and gamma rays. The two smaller nuclei are the ''fission products''. (See also Fission products (by element)). About 0.2% to 0.4% of fissions are ternary fissions, producing a third light nucleus such as helium-4 (90%) or tritium (7%). The fission products themselves are usually unstable and therefore radioactive. Due to being relatively neutron-rich for their atomic number, many of them quickly undergo beta decay. This releases additional energy in the form of beta particles, antineutrinos, and gamma rays. Thus, fission events normally result in beta and gamma radiation, even though this radiation is not produced directly by the fission event itself. The produced radionuclides have varyi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have almost the same chemical properties, they have different atomic masses and physical properties. The term isotope is formed from the Greek roots isos ( ἴσος "equal") and topos ( τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd in 1913 in a suggestion to the British chemist Frederick Soddy. The number of protons within the atom's nucleus is called its atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic numbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Civil Defense
Civil defense ( en, region=gb, civil defence) or civil protection is an effort to protect the citizens of a state (generally non-combatants) from man-made and natural disasters. It uses the principles of emergency operations: prevention, mitigation, preparation, response, or emergency evacuation and recovery. Programs of this sort were initially discussed at least as early as the 1920s and were implemented in some countries during the 1930s as the threat of war and aerial bombardment grew. Civil-defense structures became widespread after authorities recognised the threats posed by nuclear weapons. Since the end of the Cold War, the focus of civil defense has largely shifted from responding to military attack to dealing with emergencies and disasters in general. The new concept is characterised by a number of terms, each of which has its own specific shade of meaning, such as ''crisis management'', '' emergency management'', ''emergency preparedness'', ''contingency planning' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine-129
Iodine-129 (129I) is a long-lived radioisotope of iodine which occurs naturally, but also is of special interest in the monitoring and effects of man-made nuclear fission products, where it serves as both tracer and potential radiological contaminant. Formation and decay 129I is one of seven long-lived fission products. It is primarily formed from the fission of uranium and plutonium in nuclear reactors. Significant amounts were released into the atmosphere as a result of nuclear weapons testing in the 1950s and 1960s. It is also naturally produced in small quantities, due to the spontaneous fission of natural uranium, by cosmic ray spallation of trace levels of xenon in the atmosphere, and by cosmic ray muons striking tellurium-130. 129I decays with a half-life of 15.7 million years, with low-energy beta and gamma emissions, to stable xenon-129 (129Xe). Fission product 129I is one of the seven long-lived fission products that are produced in significant amounts. Its yield ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spontaneous Fission
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 56 (e.g., iron-56); spontaneous breakdown into smaller nuclei and a few isolated nuclear particles becomes possible at greater atomic mass numbers. History By 1908, physicists understood that alpha decay involved ejection of helium nuclei from a decaying atom. Like cluster decay, alpha decay is not typically categorized as a process of fission. The first nuclear fission process discovered was fission induced by neutrons. Because cosmic rays produce some neutrons, it was difficult to distinguish between induced and spontaneous events. Cosmic rays can be reliably shielded by a thick layer of rock or water. Spontaneous fission was identified in 1940 by Soviet physicists Georgy Flyorov and Konstantin Petrzhak by their observations of uranium in the Moscow Metro Dinamo station ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. This process thereby changes a nuclear proton to a neutron and simultaneously causes the emission of an electron neutrino. : : or when written as a nuclear reaction equation, ^_e + ^_p -> ^_n + ^_ ν_e Since this single emitted neutrino carries the entire decay energy, it has this single characteristic energy. Similarly, the momentum of the neutrino emission causes the daughter atom to recoil with a single characteristic momentum. The resulting daughter nuclide, if it is in an excited state, then transitions to its ground state. Usually, a gamma ray is emitted during this transition, but nuclear de-excitation may also take place by internal conversion. Following capture of an inner electron from the atom, an outer electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine-125
Iodine-125 (125I) is a radioisotope of iodine which has uses in biological assays, nuclear medicine imaging and in radiation therapy as brachytherapy to treat a number of conditions, including prostate cancer, uveal melanomas, and brain tumors. It is the second longest-lived radioisotope of iodine, after iodine-129. Its half-life is 59.49 days and it decays by electron capture to an excited state of tellurium-125. This state is not the metastable 125mTe, but rather a lower energy state that decays immediately by gamma decay with a maximum energy of 35 keV. Some of the excess energy of the excited 125Te may be internally converted ejected electrons (also at 35 keV), or to x-rays (from electron bremsstrahlung), and also a total of 21 Auger electrons, which are produced at the low energies of 50 to 500 electron volts. Eventually, stable ground state 125Te is produced as the final decay product. In medical applications, the internal conversion and Auger electrons cause little dam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Medicine
Nuclear medicine or nucleology is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging, in a sense, is "radiology done inside out" because it records radiation emitting from within the body rather than radiation that is generated by external sources like X-rays. In addition, nuclear medicine scans differ from radiology, as the emphasis is not on imaging anatomy, but on the function. For such reason, it is called a physiological imaging modality. Single photon emission computed tomography (SPECT) and positron emission tomography (PET) scans are the two most common imaging modalities in nuclear medicine. Diagnostic medical imaging Diagnostic In nuclear medicine imaging, radiopharmaceuticals are taken internally, for example, through inhalation, intravenously or orally. Then, external detectors (gamma cameras) capture and form images from the radiation emitted by the radiopharmaceuticals. This process ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine-123
Iodine-123 (123I) is a radioactive isotope of iodine used in nuclear medicine imaging, including single-photon emission computed tomography (SPECT) or SPECT/CT exams. The isotope's half-life is 13.2230 hours; the decay by electron capture to tellurium-123 emits gamma radiation with a predominant energy of 159 keV (this is the gamma primarily used for imaging). In medical applications, the radiation is detected by a gamma camera. The isotope is typically applied as iodide-123, the anionic form. Production Iodine-123 is produced in a cyclotron by proton irradiation of xenon in a capsule. Xenon-124 absorbs a proton and immediately loses a neutron and proton to form xenon-123, or else loses two neutrons to form caesium-123, which decays to xenon-123. The xenon-123 formed by either route then decays to iodine-123, and is trapped on the inner wall of the irradiation capsule under refrigeration, then eluted with sodium hydroxide in a halogen disproportionation reaction, similar to colle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
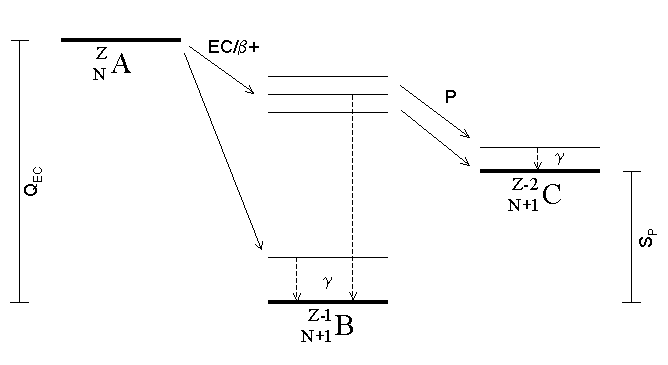
Proton Emission
Proton emission (also known as proton radioactivity) is a rare type of radioactive decay in which a proton is ejected from a nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay, in which case the process is known as beta-delayed proton emission, or can occur from the ground state (or a low-lying isomer) of very proton-rich nuclei, in which case the process is very similar to alpha decay. For a proton to escape a nucleus, the proton separation energy must be negative—the proton is therefore unbound, and tunnels out of the nucleus in a finite time. Proton emission is not seen in naturally occurring isotopes; proton emitters can be produced via nuclear reactions, usually using linear particle accelerators. Although prompt (i.e. not beta-delayed) proton emission was observed from an isomer in cobalt-53 as early as 1969, no other proton-emitting states were found until 1981, when the proton radioactive ground states of lutetium-15 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_Halflife_of_Radionulides_depending_on_Z²_to_A_ratio.png)