|
Rydberg State
The Rydberg states of an atom or molecule are electronically excited states with energies that follow the Rydberg formula as they converge on an ionic state with an ionization energy. Although the Rydberg formula was developed to describe atomic energy levels, it has been used to describe many other systems that have electronic structure roughly similar to atomic hydrogen. In general, at sufficiently high principal quantum numbers, an excited electron-ionic core system will have the general character of a hydrogenic system and the energy levels will follow the Rydberg formula. Rydberg states have energies converging on the energy of the ion. The ionization energy threshold is the energy required to completely liberate an electron from the ionic core of an atom or molecule. In practice, a Rydberg wave packet is created by a laser pulse on a hydrogenic atom and thus populates a superposition of Rydberg states. Modern investigations using pump-probe experiments show molecular pathways ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
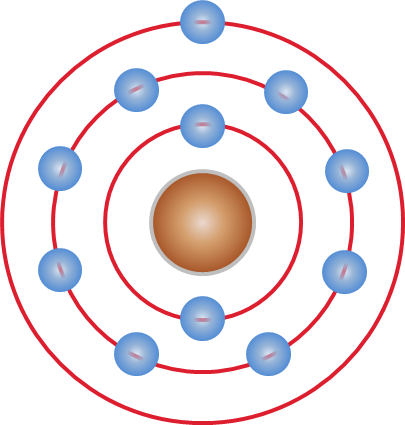
Atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics, as if they were tennis balls for example, is not possible due to quantum effects. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions. The electrons of an at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Niels Bohr
Niels Henrik David Bohr (; 7 October 1885 – 18 November 1962) was a Danish physicist who made foundational contributions to understanding atomic structure and quantum theory, for which he received the Nobel Prize in Physics in 1922. Bohr was also a philosopher and a promoter of scientific research. Bohr developed the Bohr model of the atom, in which he proposed that energy levels of electrons are discrete and that the electrons revolve in stable orbits around the atomic nucleus but can jump from one energy level (or orbit) to another. Although the Bohr model has been supplanted by other models, its underlying principles remain valid. He conceived the principle of complementarity: that items could be separately analysed in terms of contradictory properties, like behaving as a wave or a stream of particles. The notion of complementarity dominated Bohr's thinking in both science and philosophy. Bohr founded the Institute of Theoretical Physics at the University of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy Level
A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can also refer to energy levels of nuclei or vibrational or rotational energy levels in molecules. The energy spectrum of a system with such discrete energy levels is said to be quantized. In chemistry and atomic physics, an electron shell, or principal energy level, may be thought of as the orbit of one or more electrons around an atom's nucleus. The closest shell to the nucleus is called the " shell" (also called "K shell"), followed by the " shell" (or "L shell"), then the " shell" (or "M shell"), and so on farther and farther from the nucleus. The shells correspond with the principal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rydberg Matter
Rydberg matter is an exotic phase of matter formed by Rydberg atoms; it was predicted around 1980 by É. A. Manykin, M. I. Ozhovan and P. P. Poluéktov. It has been formed from various elements like caesium, potassium, hydrogen and nitrogen; studies have been conducted on theoretical possibilities like sodium, beryllium, magnesium and calcium. It has been suggested to be a material that diffuse interstellar bands may arise from. Circular Rydberg states, where the outermost electron is found in a planar circular orbit, are the most long-lived, with lifetimes of up to several hours, and are the most common. Physical Rydberg matter consists of usually hexagonal planar clusters; these cannot be very big because of the retardation effect caused by the finite velocity of the speed of light. Hence, they are not gases or plasmas; nor are they solids or liquids; they are most similar to dusty plasmas with small clusters in a gas. Though Rydberg matter can be studied in the laboratory b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rydberg Atom
A Rydberg atom is an excited atom with one or more electrons that have a very high principal quantum number, ''n''. The higher the value of ''n'', the farther the electron is from the nucleus, on average. Rydberg atoms have a number of peculiar properties including an exaggerated response to electric and magnetic fields, long decay periods and electron wavefunctions that approximate, under some conditions, classical orbits of electrons about the nuclei. The core electrons shield the outer electron from the electric field of the nucleus such that, from a distance, the electric potential looks identical to that experienced by the electron in a hydrogen atom. In spite of its shortcomings, the Bohr model of the atom is useful in explaining these properties. Classically, an electron in a circular orbit of radius ''r'', about a hydrogen nucleus of charge +'' e'', obeys Newton's second law: : \mathbf=m\mathbf \Rightarrow = where ''k'' = 1/(4π ε0). Orbital momentum is quantiz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monovalent Ion
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Description The combining capacity, or affinity of an atom of a given element is determined by the number of hydrogen atoms that it combines with. In methane, carbon has a valence of 4; in ammonia, nitrogen has a valence of 3; in water, oxygen has a valence of 2; and in hydrogen chloride, chlorine has a valence of 1. Chlorine, as it has a valence of one, can be substituted for hydrogen. Phosphorus has a valence of 5 in phosphorus pentachloride, . Valence diagrams of a compound represent the connectivity of the elements, with lines drawn between two elements, sometimes called bonds, representing a saturated valency for each element. The two tables below show some examples of different compounds, their valence diagrams, and the valences for each element of the compound. Modern definitions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wave Functions
A wave function in quantum physics is a mathematical description of the quantum state of an isolated quantum system. The wave function is a complex-valued probability amplitude, and the probabilities for the possible results of measurements made on the system can be derived from it. The most common symbols for a wave function are the Greek letters and (lower-case and capital psi, respectively). The wave function is a function of the degrees of freedom corresponding to some maximal set of commuting observables. Once such a representation is chosen, the wave function can be derived from the quantum state. For a given system, the choice of which commuting degrees of freedom to use is not unique, and correspondingly the domain of the wave function is also not unique. For instance, it may be taken to be a function of all the position coordinates of the particles over position space, or the momenta of all the particles over momentum space; the two are related by a Fourier tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ratio). Protons and neutrons, each with masses of approximately one atomic mass unit, are jointly referred to as "nucleons" (particles present in atomic nuclei). One or more protons are present in the Atomic nucleus, nucleus of every atom. They provide the attractive electrostatic central force which binds the atomic electrons. The number of protons in the nucleus is the defining property of an element, and is referred to as the atomic number (represented by the symbol ''Z''). Since each chemical element, element has a unique number of protons, each element has its own unique atomic number, which determines the number of atomic electrons and consequently the chemical characteristics of the element. The word ''proton'' is Greek language, G ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rydberg Correction
The term quantum defect refers to two concepts: energy loss in lasers and energy levels in alkali elements. Both deal with quantum systems where matter interacts with light. In laser science In laser science, the term "quantum defect" refers to the fact that the energy of a pump photon is generally higher than that of a ''signal photon'' (photon of the output radiation). The energy difference is lost to heat, which may carry away the excess entropy delivered by the multimode incoherent pump. The quantum defect of a laser can be defined as part of the energy of the pumping photon, which is lost (not turned into photons at the lasing wavelength) in the gain medium at the lasing. At given frequency \omega_ of pump and given frequency \omega_ of lasing, the quantum defect q = \hbar \omega_ - \hbar\omega_. Such quantum defect has dimension of energy; for the efficient operation, the temperature of the gain medium (measured in units of energy) should be small compared to the quantum de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of heat and light. Energy is a conserved quantity—the law of conservation of energy states that energy can be converted in form, but not created or destroyed. The unit of measurement for energy in the International System of Units (SI) is the joule (J). Common forms of energy include the kinetic energy of a moving object, the potential energy stored by an object (for instance due to its position in a field), the elastic energy stored in a solid object, chemical energy associated with chemical reactions, the radiant energy carried by electromagnetic radiation, and the internal energy contained within a thermodynamic system. All living organisms constantly take in and release energy. Due to mass–energy equivalence, any object that h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |