|
Ruthenium(IV) Oxide
Ruthenium(IV) oxide is the inorganic compound with the formula Ru O2. This black solid is the most common oxide of ruthenium. It is widely used as an electrocatalyst for producing chlorine, chlorine oxides, and O2. Like many dioxides, RuO2 adopts the rutile structure. Preparation It is usually prepared by oxidation of ruthenium trichloride. Nearly stoichiometric single crystals of RuO2 can be obtained by chemical vapor transport, using O2 as the transport agent: :RuO2 + O2 RuO4 Films of RuO2 can be prepared by chemical vapor deposition (CVD) from volatile ruthenium compounds. RuO2 can also be prepared through electroplating from a solution of ruthenium trichloride. Electrostatically stabilized hydrosols of pristine ruthenium dioxide hydrate have been prepared by exploiting the autocatalytic reduction of ruthenium tetroxide in aqueous solution. The resulting particle populations may be controlled to comprise substantially monodisperse, uniform spheres with diameters in t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rutile
Rutile is an oxide mineral composed of titanium dioxide (TiO2), the most common natural form of TiO2. Rarer Polymorphism (materials science), polymorphs of TiO2 are known, including anatase, akaogiite, and brookite. Rutile has one of the highest refractive index, refractive indices at visible wavelengths of any known crystal and also exhibits a particularly large birefringence and high dispersion (optics), dispersion. Owing to these properties, it is useful for the manufacture of certain optical elements, especially Polarization (waves), polarization optics, for longer light, visible and infrared, infrared wavelengths up to about 4.5 micrometres. Natural rutile may contain up to 10% iron and significant amounts of niobium and tantalum. Rutile derives its name from the Latin ('red'), in reference to the deep red color observed in some specimens when viewed by transmitted light. Rutile was first described in 1803 by Abraham Gottlob Werner. Occurrence Rutile is a common accessory ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Vapor Transport
In chemistry, a chemical transport reaction describes a process for purification and crystallization of non- volatile solids. The process is also responsible for certain aspects of mineral growth from the effluent of volcanoes. The technique is distinct from chemical vapor deposition, which usually entails decomposition of molecular precursors and which gives conformal coatings. The technique, which was popularized by Harald Schäfer, entails the reversible conversion of nonvolatile elements and chemical compounds into volatile derivatives. The volatile derivative migrates throughout a sealed reactor, typically a sealed and evacuated glass tube heated in a tube furnace. Because the tube is under a temperature gradient, the volatile derivative reverts to the parent solid and the transport agent is released at the end opposite to which it originated (see next section). The transport agent is thus catalytic. The technique requires that the two ends of the tube (which contains ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Integrated Circuits
An integrated circuit or monolithic integrated circuit (also referred to as an IC, a chip, or a microchip) is a set of electronic circuits on one small flat piece (or "chip") of semiconductor material, usually silicon. Large numbers of tiny MOSFETs (metal–oxide–semiconductor field-effect transistors) integrate into a small chip. This results in circuits that are orders of magnitude smaller, faster, and less expensive than those constructed of discrete electronic components. The IC's mass production capability, reliability, and building-block approach to integrated circuit design has ensured the rapid adoption of standardized ICs in place of designs using discrete transistors. ICs are now used in virtually all electronic equipment and have revolutionized the world of electronics. Computers, mobile phones and other home appliances are now inextricable parts of the structure of modern societies, made possible by the small size and low cost of ICs such as modern compute ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
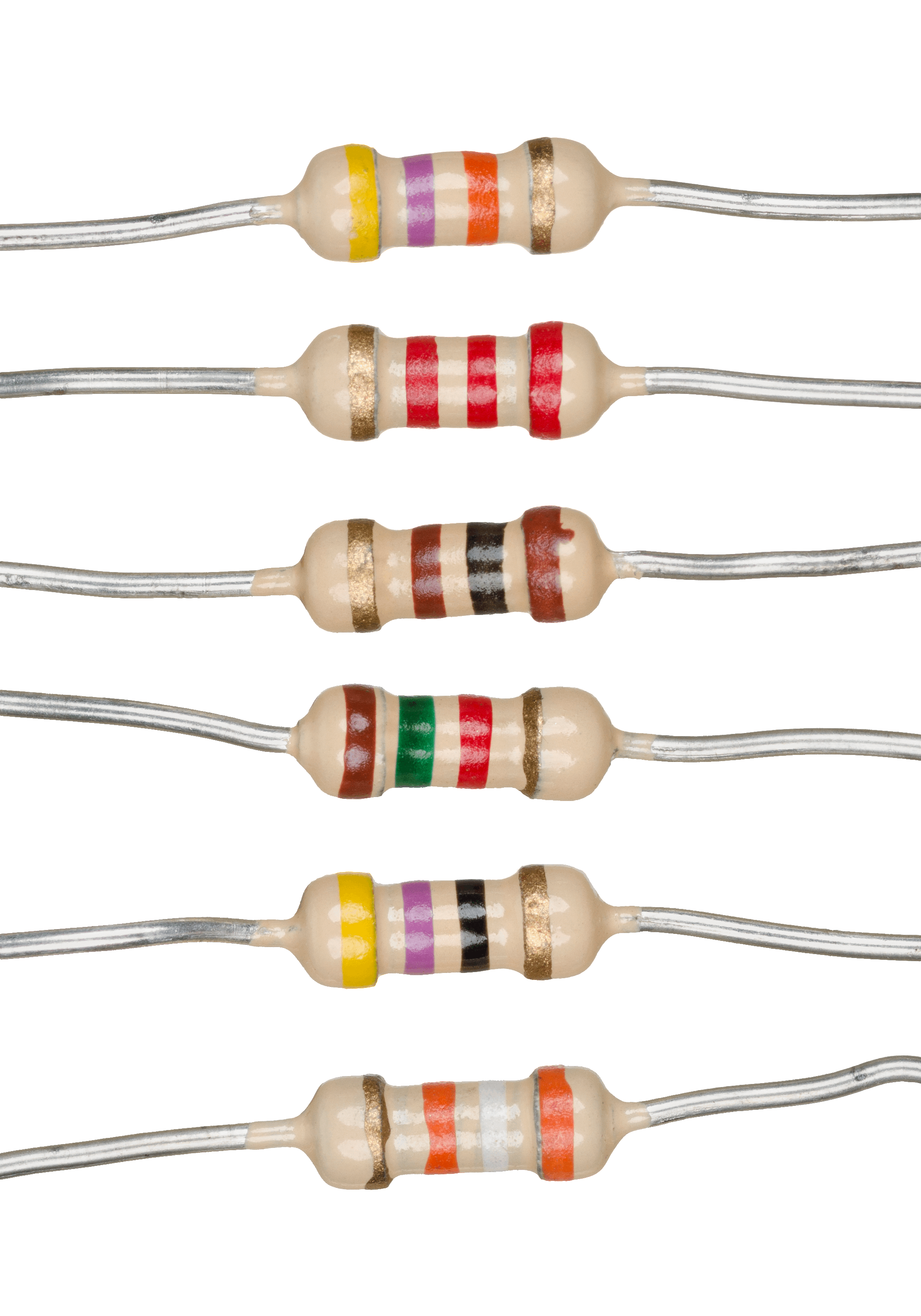
Resistors
A resistor is a passive two-terminal electrical component that implements electrical resistance as a circuit element. In electronic circuits, resistors are used to reduce current flow, adjust signal levels, to divide voltages, bias active elements, and terminate transmission lines, among other uses. High-power resistors that can dissipate many watts of electrical power as heat may be used as part of motor controls, in power distribution systems, or as test loads for generators. Fixed resistors have resistances that only change slightly with temperature, time or operating voltage. Variable resistors can be used to adjust circuit elements (such as a volume control or a lamp dimmer), or as sensing devices for heat, light, humidity, force, or chemical activity. Resistors are common elements of electrical networks and electronic circuits and are ubiquitous in electronic equipment. Practical resistors as discrete components can be composed of various compounds and forms. Resistor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fuel Cells
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen fuel, hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most battery (electricity), batteries in requiring a continuous source of fuel and oxygen (usually from air) to sustain the chemical reaction, whereas in a battery the chemical energy usually comes from substances that are already present in the battery. Fuel cells can produce electricity continuously for as long as fuel and oxygen are supplied. The first fuel cells were invented by Sir William Robert Grove, William Grove in 1838. The first commercial use of fuel cells came more than a century later following the invention of the hydrogen–oxygen fuel cell by Francis Thomas Bacon in 1932. The alkaline fuel cell, also known as the Bacon fuel cell after its inventor, has been used in NASA space programs since the mid-1960s to generate power for sat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haber–Bosch Process
The Haber process, also called the Haber–Bosch process, is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today. It is named after its inventors, the German chemists Fritz Haber and Carl Bosch, who developed it in the first decade of the 20th century. The process converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using a metal catalyst under high temperatures and pressures: : \ce \quad \Delta H^\circ = -91.8~\text Though this reaction is exothermic (i.e. it releases energy, albeit not very much), it results in a decrease in entropy, which is the central reason why it is very challenging to carry out. Before the development of the Haber process, it had been difficult to produce ammonia on an industrial scale, with early methods, such as the Birkeland–Eyde process and the Frank–Caro process, all highly inefficient. During World War I, the Haber process provided Germany with a sou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fischer–Tropsch Process
The Fischer–Tropsch process is a collection of chemical reactions that converts a mixture of carbon monoxide and hydrogen, known as syngas, into liquid hydrocarbons. These reactions occur in the presence of metal catalysts, typically at temperatures of and pressures of one to several tens of atmospheres. The process was first developed by Franz Fischer and Hans Tropsch at the Kaiser Wilhelm Institute for Coal Research in Mülheim an der Ruhr, Germany, in 1925. As a premier example of C1 chemistry, the Fischer–Tropsch process is an important reaction in both coal liquefaction and gas to liquids technology for producing liquid hydrocarbons. In the usual implementation, carbon monoxide and hydrogen, the feedstocks for FT, are produced from coal, natural gas, or biomass in a process known as gasification. The process then converts these gases into synthetic oil, synthetic lubrication oil and synthetic fuel. This process has received intermittent attention as a source of low-s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride gas and hydrochloric acid are important in technology and industry. Hydrochloric acid, the aqueous solution of hydrogen chloride, is also commonly given the formula HCl. Reactions Hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom H and a chlorine atom Cl connected by a polar covalent bond. The chlorine atom is much more electronegative than the hydrogen atom, which makes this bond polar. Consequently, the molecule has a large dipole moment with a negative partial charge (δ−) at the chlorine atom and a positive partial charge (δ+) at the hydrogen atom. In part because of its high polarity, HCl is very soluble in water (and in other polar solvents). Upon contact, and HCl combine to form hydronium cations and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and hydrochloric acid (in the form of ). However ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deacon Process
The Deacon process, invented by Henry Deacon, is a process used during the manufacture of alkalis (the initial end product was sodium carbonate) by the Leblanc process. Hydrogen chloride gas was converted to chlorine gas, which was then used to manufacture a commercially valuable bleaching powder, and at the same time the emission of waste hydrochloric acid was curtailed. To some extent this technically sophisticated process superseded the earlier manganese dioxide process. Process The process was based on the oxidation of hydrogen chloride: :4 HCl + O2 → 2 Cl2 + 2H2O The reaction takes place at about 400 to 450 °C in the presence of a variety of catalysts, including copper chloride (CuCl2). Three companies developed commercial processes for producing chlorine based on the Deacon reaction:Peter Schmittinger et al. "Chlorine," Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co, 2006, *The Kel-Chlor process developed by the M. W. K ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electroplating
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be coated acts as the cathode (negative electrode) of an electrolytic cell; the electrolyte is a solution of a salt of the metal to be coated; and the anode (positive electrode) is usually either a block of that metal, or of some inert conductive material. The current is provided by an external power supply. Electroplating is widely used in industry and decorative arts to improve the surface qualities of objects—such as resistance to abrasion and corrosion, lubricity, reflectivity, electrical conductivity, or appearance. It is used to build up thickness on undersized or worn-out parts, or to manufacture metal plates with complex shape, a process called electroforming. It is used to deposit copper and other conductors in forming printe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Vapor Deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films. In typical CVD, the wafer (substrate) is exposed to one or more volatile precursors, which react and/or decompose on the substrate surface to produce the desired deposit. Frequently, volatile by-products are also produced, which are removed by gas flow through the reaction chamber. Microfabrication processes widely use CVD to deposit materials in various forms, including: monocrystalline, polycrystalline, amorphous, and epitaxial. These materials include: silicon ( dioxide, carbide, nitride, oxynitride), carbon (fiber, nanofibers, nanotubes, diamond and graphene), fluorocarbons, filaments, tungsten, titanium nitride and various high-κ dielectrics. The term ''chemical vapour deposition'' was coined 1960 by ''John M. Blocher, Jr.'' who intended to differentiate ''chemic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)