|
Roquefortine C To Isoroquefortine C
Roquefortine C is a mycotoxin that belongs to a class of naturally occurring 2,5-diketopiperazines produced by various fungi, particularly species from the genus ''Penicillium''. It was first isolated from a strain of ''Penicillium roqueforti'', a species commercially used as a source of proteolytic and lipolytic enzymes during maturation of the blue-veined cheeses, Roquefort, Danish Blue, Stilton and Gorgonzola. Roquefortine C is a cyclodipeptide mycotoxin derived from the diketopiperazine cyclo(Trp-dehydro-His) and is a relatively common fungal metabolite produced by a number of ''Penicillium'' species. It is also considered one of the most important fungal contaminants of carbonated beverages, beer, wine, meats, cheese and bread. At high doses roquefortine C is classified as a toxic compound. Although it is a potent neurotoxin at high doses, at low concentrations of 0.05 to 1.47 mg/kg that occur in domestic cheeses, it was found to be "safe for the consumer". The mechanisms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mycotoxin
A mycotoxin (from the Greek μύκης , "fungus" and τοξίνη , "toxin") is a toxic secondary metabolite produced by organisms of kingdom Fungi and is capable of causing disease and death in both humans and other animals. The term 'mycotoxin' is usually reserved for the toxic chemical products produced by fungi that readily colonize crops. Examples of mycotoxins causing human and animal illness include aflatoxin, citrinin, fumonisins, ochratoxin A, patulin, trichothecenes, zearalenone, and ergot alkaloids such as ergotamine. One mold species may produce many different mycotoxins, and several species may produce the same mycotoxin. Production Most fungi are aerobic (use oxygen) and are found almost everywhere in extremely small quantities due to the diminute size of their spores. They consume organic matter wherever humidity and temperature are sufficient. Where conditions are right, fungi proliferate into colonies and mycotoxin levels become high. The reason for the product ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haemoprotein
A hemeprotein (or haemprotein; also hemoprotein or haemoprotein), or heme protein, is a protein that contains a heme prosthetic group. They are a very large class of metalloproteins. The heme group confers functionality, which can include oxygen carrying, oxygen reduction, electron transfer, and other processes. Heme is bound to the protein either covalently or noncovalently or both. The heme consists of iron cation bound at the center of the conjugate base of the porphyrin, as well as other ligands attached to the "axial sites" of the iron. The porphyrin ring is a planar dianionic, tetradentate ligand. The iron is typically Fe2+ or Fe3+. One or two ligands are attached at the axial sites. The porphyrin ring has 4 nitrogen atoms that bind to the iron, leaving two other coordination positions of the iron available for bonding to the histidine of the protein and a divalent atom. Hemeproteins probably evolved to incorporate the iron atom contained within the protoporphyrin IX rin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactams
A lactam is a cyclic amide, formally derived from an amino alkanoic acid. The term is a portmanteau of the words ''lactone'' + ''amide''. Nomenclature Greek prefixes in alphabetical order indicate ring size: * α-Lactam (3-atom rings) * β-Lactam (4-atom rings) * γ-Lactam (5-atom rings) * δ-Lactam (6-atom rings) * ε-Lactam (7-atom rings) This ring-size nomenclature stems from the fact that a hydrolyzed α-Lactam leads to an α-amino acid and a β-Lactam to a β-amino acid, ''etc''. Synthesis General synthetic methods exist for the organic synthesis of lactams. Beckmann rearrangement Lactams form by the acid-catalyzed rearrangement of oximes in the Beckmann rearrangement. Schmidt reaction Lactams form from cyclic ketones and hydrazoic acid in the Schmidt reaction. Cyclization of amino acids Lactams can be formed from cyclisation of amino acids via the coupling between an amine and a carboxylic acid within the same molecule. Lactamization is most efficient in this wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imidazoles
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-adjacent nitrogen atoms in meta-substitution. Many natural products, especially alkaloids, contain the imidazole ring. These imidazoles share the 1,3-C3N2 ring but feature varied substituents. This ring system is present in important biological building blocks, such as histidine and the related hormone histamine. Many drugs contain an imidazole ring, such as certain antifungal drugs, the nitroimidazole series of antibiotics, and the sedative midazolam. When fused to a pyrimidine ring, it forms a purine, which is the most widely occurring nitrogen-containing heterocycle in nature. The name "imidazole" was coined in 1887 by the German chemist Arthur Rudolf Hantzsch (1857–1935). Structure and properties Imidazole is a planar 5-membered ri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mycotoxins
A mycotoxin (from the Greek μύκης , "fungus" and τοξίνη , "toxin") is a toxic secondary metabolite produced by organisms of kingdom Fungi and is capable of causing disease and death in both humans and other animals. The term 'mycotoxin' is usually reserved for the toxic chemical products produced by fungi that readily colonize crops. Examples of mycotoxins causing human and animal illness include aflatoxin, citrinin, fumonisins, ochratoxin A, patulin, trichothecenes, zearalenone, and ergot alkaloids such as ergotamine. One mold species may produce many different mycotoxins, and several species may produce the same mycotoxin. Production Most fungi are aerobic (use oxygen) and are found almost everywhere in extremely small quantities due to the diminute size of their spores. They consume organic matter wherever humidity and temperature are sufficient. Where conditions are right, fungi proliferate into colonies and mycotoxin levels become high. The reason for the product ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Patulin
Patulin is an organic compound classified as a polyketide. It is a white powder soluble in acidic water and in organic solvents. It is a lactone that is heat-stable, so it is not destroyed by pasteurization or thermal denaturation.http://www.sigmaaldrich.com/catalog/product/sigma/p1639?lang=en®ion=US However, stability following fermentation is lessened. It is a mycotoxin produced by a variety of molds, in particular, ''Aspergillus'' and ''Penicillium'' and '' Byssochlamys''. Most commonly found in rotting apples, the amount of patulin in apple products is generally viewed as a measure of the quality of the apples used in production. In addition, patulin has been found in other foods such as grains, fruits, and vegetables. Its presence is highly regulated. Biosynthesis, synthesis, and reactivity The immediate precursor is 6-methylsalicylic acid. Isoepoxydon dehydrogenase (IDH) is an important enzyme in the multi-step biosynthesis of patulin. Its gene is present in other fu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aflatoxin
Aflatoxins are various poisonous carcinogens and mutagens that are produced by certain molds, particularly ''Aspergillus'' species. The fungi grow in soil, decaying vegetation and various staple foodstuffs and commodities such as hay, sweetcorn, wheat, millet, sorghum, cassava, rice, chili peppers, cottonseed, peanuts, tree nuts, sesame seeds, sunflower seeds, and various spices. In short, the relevant fungi grow on almost any crop or food. When such contaminated food is processed or consumed, the aflatoxins enter the general food supply. They have been found in both pet and human foods, as well as in feedstocks for agricultural animals. Animals fed contaminated food can pass aflatoxin transformation products into eggs, milk products, and meat. For example, contaminated poultry feed is the suspected source of aflatoxin-contaminated chicken meat and eggs in Pakistan. Children are particularly affected by aflatoxin exposure, which is associated with stunted growth, delayed de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aristolochene
Aristolochene is a bicyclic sesquiterpene produced by certain fungi including the cheese mold '' Penicillium roqueforti''. It is biosynthesized from farnesyl pyrophosphate by aristolochene synthase and is the parent hydrocarbon of a large variety of fungal toxins. , Chem 549, College of Pharmacy, University of Arizona The substance was first isolated from '' Penicillium roqueforti'', a fungus used to make blue cheeses like , , [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Roquefortine C To Isoroquefortine C
Roquefortine C is a mycotoxin that belongs to a class of naturally occurring 2,5-diketopiperazines produced by various fungi, particularly species from the genus ''Penicillium''. It was first isolated from a strain of ''Penicillium roqueforti'', a species commercially used as a source of proteolytic and lipolytic enzymes during maturation of the blue-veined cheeses, Roquefort, Danish Blue, Stilton and Gorgonzola. Roquefortine C is a cyclodipeptide mycotoxin derived from the diketopiperazine cyclo(Trp-dehydro-His) and is a relatively common fungal metabolite produced by a number of ''Penicillium'' species. It is also considered one of the most important fungal contaminants of carbonated beverages, beer, wine, meats, cheese and bread. At high doses roquefortine C is classified as a toxic compound. Although it is a potent neurotoxin at high doses, at low concentrations of 0.05 to 1.47 mg/kg that occur in domestic cheeses, it was found to be "safe for the consumer". The mechanisms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomerization. When the isomerization occurs intramolecularly it may be called a rearrangement reaction. When the activation energy for the isomerization reaction is sufficiently small, both isomers will exist in a temperature-dependent equilibrium with each other. Many values of the standard free energy difference, \Delta G^\circ, have been calculated, with good agreement between observed and calculated data. Examples and applications Alkanes Skeletal isomerization occurs in the cracking process, used in the petrochemical industry. As well as reducing the average chain length, straight-chain hydrocarbons are converted to branched isomers in the process, as illustrated the following reaction of ''n''-butane to ''i''-butane. :\overset -> \o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
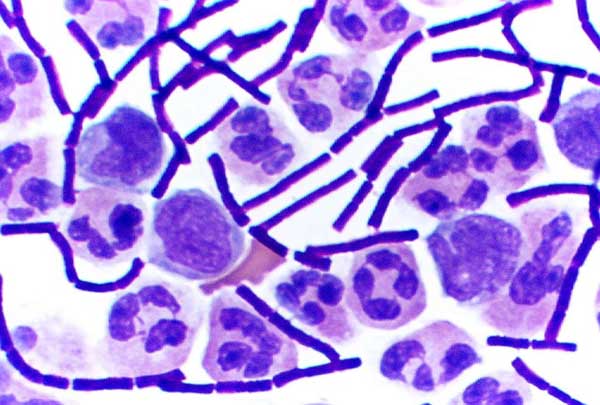
Gram-positive Bacteria
In bacteriology, gram-positive bacteria are bacteria that give a positive result in the Gram stain test, which is traditionally used to quickly classify bacteria into two broad categories according to their type of cell wall. Gram-positive bacteria take up the crystal violet stain used in the test, and then appear to be purple-coloured when seen through an optical microscope. This is because the thick peptidoglycan layer in the bacterial cell wall retains the stain after it is washed away from the rest of the sample, in the decolorization stage of the test. Conversely, gram-negative bacteria cannot retain the violet stain after the decolorization step; alcohol used in this stage degrades the outer membrane of gram-negative cells, making the cell wall more porous and incapable of retaining the crystal violet stain. Their peptidoglycan layer is much thinner and sandwiched between an inner cell membrane and a bacterial outer membrane, causing them to take up the counterstain (sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |