|
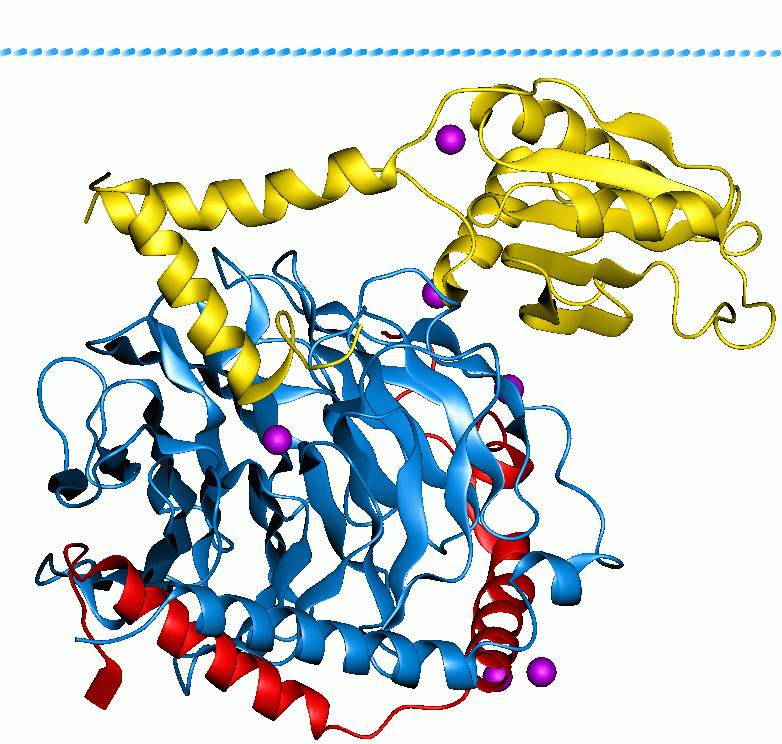
Rock1079
ROCK1 is a protein serine/threonine kinase also known as rho-associated, coiled-coil-containing protein kinase 1. Other common names are ROKβ and P160ROCK. ROCK1 is a major downstream effecter of the small GTPase RhoA and is a regulator of the actomyosin cytoskeleton which promotes contractile force generation. ROCK1 plays a role in cancer and in particular cell motility, metastasis, and angiogenesis. Gene and expression ROCK1 is also the name of the gene that encodes the protein ROCK1, a serine/threonine kinase. ROCK1 is activated when bound to the GTP-bound form of RhoA. The human ROCK1 gene is located on human chromosome 18 with specific location of 18q11.1. The location of the base pair starts at 18,529,703 and ends at 18,691,812 bp and translates into 1354 amino acids. ROCK1 has a ubiquitous tissue distribution, but subcellularly it is thought to colocalize with the centrosomes. This is consistent with its function as a key modulator of cell motility, tumor cell inva ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine/threonine Kinase
A serine/threonine protein kinase () is a kinase enzyme, in particular a protein kinase, that phosphorylates the OH group of the amino-acid residues serine or threonine, which have similar side chains. At least 350 of the 500+ human protein kinases are serine/threonine kinases (STK). In enzymology, the term ''serine/threonine protein kinase'' describes a class of enzymes in the family of transferases, that transfer phosphates to the oxygen atom of a serine or threonine side chain in proteins. This process is called phosphorylation. Protein phosphorylation in particular plays a significant role in a wide range of cellular processes and is a very important posttranslational modification. The chemical reaction performed by these enzymes can be written as :ATP + a protein \rightleftharpoons ADP + a phosphoprotein Thus, the two substrates of this enzyme are ATP and a protein, whereas its two products are ADP and phosphoprotein. The systematic name of this enzyme class is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coiled-coil
A coiled coil is a structural motif in proteins in which 2–7 alpha helix, alpha-helices are coiled together like the strands of a rope. (Protein dimer, Dimers and Protein trimer, trimers are the most common types.) Many coiled coil-type proteins are involved in important biological functions, such as the regulation of gene expression — e.g., transcription factors. Notable examples are the oncoproteins c-Fos and c-Jun, as well as the muscle protein tropomyosin. Discovery The possibility of coiled coils for α-keratin was initially somewhat controversial. Linus Pauling and Francis Crick independently came to the conclusion that this was possible at about the same time. In the summer of 1952, Pauling visited the laboratory in England where Crick worked. Pauling and Crick met and spoke about various topics; at one point, Crick asked whether Pauling had considered "coiled coils" (Crick came up with the term), to which Pauling said he had. Upon returning to the United States, Paul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rnd3
Rnd3 is a small (~21 kDa) signaling G protein (to be specific, a GTPase), and is a member of the Rnd subgroup of the Rho family of GTPases. It is encoded by the gene RND3. Like other members of the Rho family of Ras-related GTPases it regulates the organization of the actin cytoskeleton in response to extracellular growth factors. Regulation Most Rho family members cycle between an inactive GDP-bound form and an active GTP-bound form. However, members of the Rnd subgroup of the Rho family are exceptions to this, binding detectably only to GTP, while having low GTPase activity, if any. Instead, Rnd family proteins are regulated through other mechanisms that control their production, degradation, phosphorylation, and localization. Interactions In its GTP-bound form, RhoA exposes regions that allow it to interact with downstream targets. Rnd3 contains a region which is similar to the one RhoA exposes for interaction with ROCK1, allowing Rnd3 to compete with RhoA for interac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Protein
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases. There are two classes of G proteins. The first function as monomeric small GTPases (small G-proteins), while the second function as heterotrimeric G protein complexes. The latter class of complexes is made up of '' alpha'' (α), ''beta'' (β) and ''gamma'' (γ) subunits. In addition, the beta and gamma subunits can form a stable dimeric complex referred to as the beta-gamma complex . Heterotrimeric G proteins located within the cell are activ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sphingomyelin
Sphingomyelin (SPH, ˌsfɪŋɡoˈmaɪəlɪn) is a type of sphingolipid found in animal cell membranes, especially in the membranous myelin sheath that surrounds some nerve cell axons. It usually consists of phosphocholine and ceramide, or a ethanolamine, phosphoethanolamine head group; therefore, sphingomyelins can also be classified as sphingophospholipids. In humans, SPH represents ~85% of all sphingolipids, and typically make up 10–20 mol % of plasma membrane lipids. Sphingomyelin was first isolated by Germans, German chemist Johann Ludwig Wilhelm Thudichum, Johann L.W. Thudicum in the 1880s. The structure of sphingomyelin was first reported in 1927 as N-acyl-sphingosine-1-phosphorylcholine. Sphingomyelin content in mammals ranges from 2 to 15% in most tissues, with higher concentrations found in nerve tissues, red blood cells, and the ocular lenses. Sphingomyelin has significant structural and functional roles in the cell. It is a plasma membrane component and parti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arachidonic Acid
Arachidonic acid (AA, sometimes ARA) is a polyunsaturated omega-6 fatty acid 20:4(ω-6), or 20:4(5,8,11,14). It is structurally related to the saturated arachidic acid found in cupuaçu butter. Its name derives from the New Latin word ''arachis'' (peanut), but peanut oil does not contain any arachidonic acid. Chemistry In chemical structure, arachidonic acid is a carboxylic acid with a 20-carbon chain and four ''cis''-double bonds; the first double bond is located at the sixth carbon from the omega end. Some chemistry sources define 'arachidonic acid' to designate any of the eicosatetraenoic acids. However, almost all writings in biology, medicine, and nutrition limit the term to ''all cis''-5,8,11,14-eicosatetraenoic acid. Biology Arachidonic acid is a polyunsaturated fatty acid present in the phospholipids (especially phosphatidylethanolamine, phosphatidylcholine, and phosphatidylinositides) of membranes of the body's cells, and is abundant in the brain, muscles, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myosin Light Chain
A myosin light chain is a light chain (small polypeptide subunit) of myosin. Myosin light chains were discovered by Chinese biochemist Cao Tianqin (Tien-chin Tsao) when he was a graduate student at the University of Cambridge in England. Structure and function Myosin light chain classes Structurally, myosin light chains belong to the EF-hand family, a large family of Ca2+- binding proteins. MLCs contain two Ca2+ - binding EF-hand motifs. MLCs isoforms modulate the Ca2+of force transduction and cross-bridge kinetics. Myosin light chains (MLCs) can be broadly classified into two groups: * Essential or alkali MLC (MLC1 or ELC), * Regulatory MLC (MLC2 or RLC). Essential and regulatory MLCs have molecular masses of 22 and 19 kDa, respectively. Structurally, MLC2 contains a serine residue that is lacking in MLC1. The presence of this amino acids allows the regulation of the conformational changes (from compacted to an elongated form) by a Ca2+-mediated phosphorylation mechanism ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorylation
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. Protein phosphorylation often activates (or deactivates) many enzymes. Glucose Phosphorylation of sugars is often the first stage in their catabolism. Phosphorylation allows cells to accumulate sugars because the phosphate group prevents the molecules from diffusing back across their transporter. Phosphorylation of glucose is a key reaction in sugar metabolism. The chemical equation for the conversion of D-glucose to D-glucose-6-phosphate in the first step of glycolysis is given by :D-glucose + ATP → D-glucose-6-phosphate + ADP : ΔG° = −16.7 kJ/mol (° indicates measurement at standard condition) Hepatic cells are freely permeable to glucose, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Dimer
In biochemistry, a protein dimer is a macromolecular complex formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ''dimer'' has roots meaning "two parts", '' di-'' + '' -mer''. A protein dimer is a type of protein quaternary structure. A protein homodimer is formed by two identical proteins. A protein heterodimer is formed by two different proteins. Most protein dimers in biochemistry are not connected by covalent bonds. An example of a non-covalent heterodimer is the enzyme reverse transcriptase, which is composed of two different amino acid chains. An exception is dimers that are linked by disulfide bridges such as the homodimeric protein NEMO. Some proteins contain specialized domains to ensure dimerization (dimerization domains) and specificity. The G protein-coupled cannabinoid receptors have the ability to form both homo- and heterodimers with several ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, DNA fragmentation, and mRNA decay. The average adult human loses between 50 and 70 billion cells each day due to apoptosis. For an average human child between eight and fourteen years old, approximately twenty to thirty billion cells die per day. In contrast to necrosis, which is a form of traumatic cell death that results from acute cellular injury, apoptosis is a highly regulated and controlled process that confers advantages during an organism's life cycle. For example, the separation of fingers and toes in a developing human embryo occurs because cells between the digits undergo apoptosis. Unlike necrosis, apoptosis produces cell fragments called apoptotic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caspase-3
Caspase-3 is a caspase protein that interacts with caspase-8 and caspase-9. It is encoded by the ''CASP3'' gene. ''CASP3'' orthologs have been identified in numerous mammals for which complete genome data are available. Unique orthologs are also present in birds, lizards, lissamphibians, and teleosts. The CASP3 protein is a member of the cysteine-aspartic acid protease (caspase) family. Sequential activation of caspases plays a central role in the execution-phase of cell apoptosis. Caspases exist as inactive proenzymes that undergo proteolytic processing at conserved aspartic residues to produce two subunits, large and small, that dimerize to form the active enzyme. This protein cleaves and activates caspases 6 and 7; and the protein itself is processed and activated by caspases 8, 9, and 10. It is the predominant caspase involved in the cleavage of amyloid-beta 4A precursor protein, which is associated with neuronal death in Alzheimer's disease. Alternative splicing of this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |