|
Response Rate (medicine)
Clinical endpoints or clinical outcomes are outcome measures referring to occurrence of disease, symptom, sign or laboratory abnormality constituting a target outcome in clinical research trials. The term may also refer to any disease or sign that strongly motivates withdrawal of an individual or entity from the trial, then often termed a ''humane (clinical) endpoint''. The primary endpoint of a clinical trial is the endpoint for which the trial is powered. Secondary endpoints are additional endpoints, preferably also pre-specified, for which the trial may not be powered. Surrogate endpoints are trial endpoints that have outcomes that substitute for a clinical endpoint, often because studying the clinical endpoint is difficult, for example using an increase in blood pressure as a surrogate for death by cardiovascular disease, where strong evidence of a causal link exists. Scope In a general sense, a clinical endpoint is included in the entities of interest in a trial. The resu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Outcome Measure
An outcome measure, endpoint, effect measure or measure of effect is a measure within medical practice or research, (primarily clinical trials) which is used to assess the effect, both positive and negative, of an intervention or treatment. Measures can often be quantified using effect sizes. Outcomes measures can be Patient-reported outcome, patient-reported, or gathered through laboratory tests such as Blood test, blood work, Clinical urine tests, urine samples etc. or through Physical examination, medical examination. Outcomes measures should be relevant to the target of the intervention (be it a single person or a target population). Depending on the design of a trial, outcome measures can be either primary outcomes, in which case the trial is designed around finding an adequate study size (through proper Random assignment, randomization and Statistical power, power calculation). Secondary or tertiary outcomes are outcome measures which are added after the design of the study is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Positron Emission Tomography
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in Metabolism, metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body. For example, 18F-FDG, -FDG is commonly used to detect cancer, Sodium fluoride#Medical imaging, NaF is widely used for detecting bone formation, and Isotopes of oxygen#Oxygen-15, oxygen-15 is sometimes used to measure blood flow. PET is a common medical imaging, imaging technique, a Scintigraphy#Process, medical scintillography technique used in nuclear medicine. A radiopharmaceutical, radiopharmaceutical — a radioisotope attached to a drug — is injected into the body as a radioactive tracer, tracer. When the radiopharmaceutical undergoes beta plus decay, a positron is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Research
Clinical research is a branch of healthcare science that determines the safety and effectiveness ( efficacy) of medications, devices, diagnostic products and treatment regimens intended for human use. These may be used for prevention, treatment, diagnosis or for relieving symptoms of a disease. Clinical research is different from clinical practice. In clinical practice established treatments are used, while in clinical research evidence is collected to establish a treatment. Overview The term "clinical research" refers to the entire bibliography of a drug/device/biologic, in fact any test article from its inception in the lab to its introduction to the consumer market and beyond. Once the promising candidate or the molecule is identified in the lab, it is subjected to pre-clinical studies or animal studies where different aspects of the test article (including its safety toxicity if applicable and efficacy, if possible at this early stage) are studied. In the United States ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immune-related Response Criteria
The immune-related response criteria (irRC) is a set of published rules that define when tumors in cancer patients improve ("respond"), stay the same ("stabilize"), or worsen ("progress") during treatment, where the compound being evaluated is an immuno-oncology drug. Immuno-oncology, part of the broader field of cancer immunotherapy, involves agents which harness the body's own immune system to fight cancer. Traditionally, patient responses to new cancer treatments have been evaluated using two sets of criteria, the WHO criteria and the response evaluation criteria in solid tumors (RECIST). The immune-related response criteria, first published in 2009, arose out of observations that immuno-oncology drugs would fail in clinical trials that measured responses using the WHO or RECIST Criteria, because these criteria could not account for the time gap in many patients between initial treatment and the apparent action of the immune system to reduce the tumor burden. Background Part o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
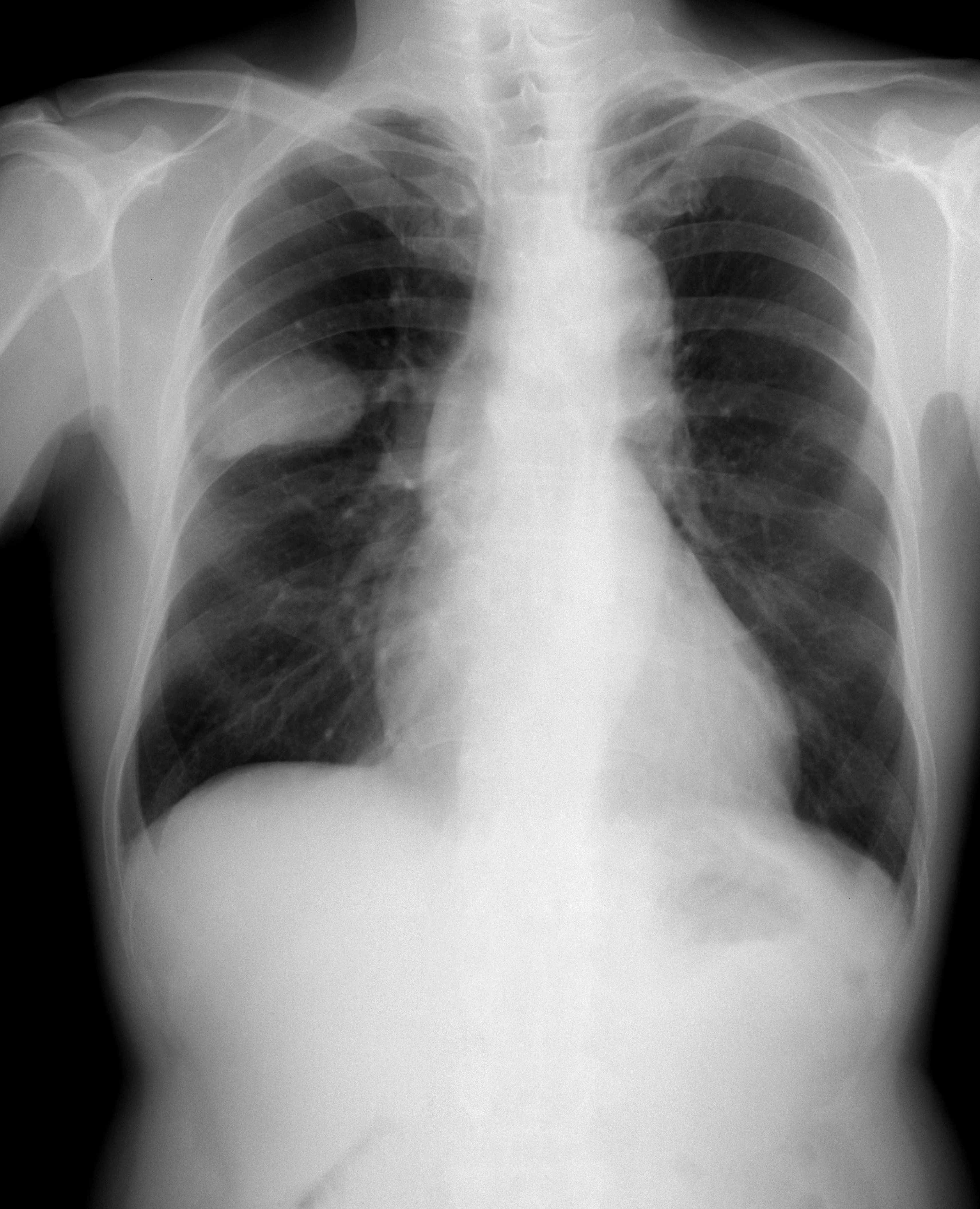
Small-cell Carcinoma
Small-cell carcinoma is a type of highly malignant cancer that most commonly arises within the lung, although it can occasionally arise in other body sites, such as the cervix, prostate, and gastrointestinal tract. Compared to non-small cell carcinoma, small cell carcinoma has a shorter doubling time, higher growth fraction, and earlier development of metastases. Extensive stage small cell lung cancer is classified as a rare disorder. Ten-year relative survival rate is 3.5%; however, women have a higher survival rate, 4.3%, and men lower, 2.8%. Survival can be higher or lower based on a combination of factors including stage, age, gender and race. Types of SCLC Small-cell lung carcinoma has long been divided into two clinicopathological stages, termed ''limited stage'' (LS) and ''extensive stage'' (ES). The stage is generally determined by the presence or absence of metastases, whether or not the tumor appears limited to the thorax, and whether or not the entire tumor burden wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
National Institutes Of Health
The National Institutes of Health, commonly referred to as NIH (with each letter pronounced individually), is the primary agency of the United States government responsible for biomedical and public health research. It was founded in the late 1880s and is now part of the United States Department of Health and Human Services. The majority of NIH facilities are located in Bethesda, Maryland, and other nearby suburbs of the Washington metropolitan area, with other primary facilities in the Research Triangle Park in North Carolina and smaller satellite facilities located around the United States. The NIH conducts its own scientific research through the NIH Intramural Research Program (IRP) and provides major biomedical research funding to non-NIH research facilities through its Extramural Research Program. , the IRP had 1,200 principal investigators and more than 4,000 postdoctoral fellows in basic, translational, and clinical research, being the largest biomedical research instit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surrogate Endpoint
In clinical trials, a surrogate endpoint (or surrogate marker) is a measure of effect of a specific treatment that may correlate with a ''real'' clinical endpoint but does not necessarily have a guaranteed relationship. The National Institutes of Health (USA) defines surrogate endpoint as "a biomarker intended to substitute for a clinical endpoint". Surrogate markers are used when the primary endpoint is undesired (e.g., death), or when the number of events is very small, thus making it impractical to conduct a clinical trial to gather a statistically significant number of endpoints. The FDA and other regulatory agencies will often accept evidence from clinical trials that show a direct clinical benefit to surrogate markers. Surrogate endpoints can be obtained from different modalities, such as, behavioural or cognitive scores, or biomarkers from Electroencephalography ( qEEG), MRI, PET, or biochemical biomarkers. A correlate does not make a surrogate. It is a common misconception ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Experimental Animal
Animal testing, also known as animal experimentation, animal research, and ''in vivo'' testing, is the use of non-human animals in experiments that seek to control the variables that affect the behavior or biological system under study. This approach can be contrasted with field studies in which animals are observed in their natural environments or habitats. Experimental research with animals is usually conducted in universities, medical schools, pharmaceutical companies, defense establishments, and commercial facilities that provide animal-testing services to the industry. The focus of animal testing varies on a continuum from pure research, focusing on developing fundamental knowledge of an organism, to applied research, which may focus on answering some questions of great practical importance, such as finding a cure for a disease. Examples of applied research include testing disease treatments, breeding, defense research, and toxicology, including cosmetics testing. In edu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C), but the agency also enforces other laws, notably Section 361 of the Public Health Service Act, as well as associated regulations. Much of this regulatory-enforcement work is not d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arithmetical Mean
In mathematics and statistics, the arithmetic mean ( ) or arithmetic average, or just the ''mean'' or the ''average'' (when the context is clear), is the sum of a collection of numbers divided by the count of numbers in the collection. The collection is often a set of results of an experiment or an observational study, or frequently a set of results from a survey. The term "arithmetic mean" is preferred in some contexts in mathematics and statistics, because it helps distinguish it from other means, such as the geometric mean and the harmonic mean. In addition to mathematics and statistics, the arithmetic mean is used frequently in many diverse fields such as economics, anthropology and history, and it is used in almost every academic field to some extent. For example, per capita income is the arithmetic average income of a nation's population. While the arithmetic mean is often used to report central tendencies, it is not a robust statistic, meaning that it is greatly influenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Median
In statistics and probability theory, the median is the value separating the higher half from the lower half of a data sample, a population, or a probability distribution. For a data set, it may be thought of as "the middle" value. The basic feature of the median in describing data compared to the mean (often simply described as the "average") is that it is not skewed by a small proportion of extremely large or small values, and therefore provides a better representation of a "typical" value. Median income, for example, may be a better way to suggest what a "typical" income is, because income distribution can be very skewed. The median is of central importance in robust statistics, as it is the most resistant statistic, having a breakdown point of 50%: so long as no more than half the data are contaminated, the median is not an arbitrarily large or small result. Finite data set of numbers The median of a finite list of numbers is the "middle" number, when those numbers are list ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)
