|
Resonance Raman Spectroscopy
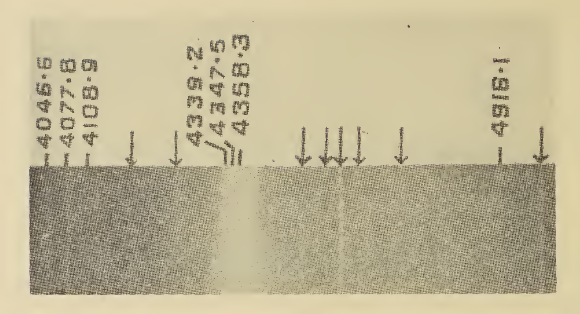
Resonance Raman spectroscopy (RR spectroscopy) is a Raman spectroscopy technique in which the incident photon energy is close in energy to an electronic transition of a compound or material under examination. The frequency coincidence (or ''resonance'') can lead to greatly enhanced intensity of the Raman scattering, which facilitates the study of chemical compounds present at low concentrations. Raman scattering is usually extremely weak, since only about 1 in 10 million photons that hit a sample are scattered with a loss ( Stokes) or gain (anti-Stokes) in energy from changes in vibrational energy of the molecules in the sample; the rest of the photons are scattered with no change in energy. Resonance enhancement of Raman scattering requires the incident wavelength (usually from a laser) to be close to that of an electronic transition of the molecules. In larger molecules the change in electron density can be largely confined to one part of the molecule, a chromophore, and in these c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Raman Spectroscopy
Raman spectroscopy () (named after Indian physicist C. V. Raman) is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman spectroscopy is commonly used in chemistry to provide a structural fingerprint by which molecules can be identified. Raman spectroscopy relies upon inelastic scattering of photons, known as Raman scattering. A source of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range is used, although X-rays can also be used. The laser light interacts with molecular vibrations, phonons or other excitations in the system, resulting in the energy of the laser photons being shifted up or down. The shift in energy gives information about the vibrational modes in the system. Infrared spectroscopy typically yields similar yet complementary information. Typically, a sample is illuminated with a laser beam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Infrared Spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance (or transmittance) on the vertical axis vs. frequency, wavenumber or wavelength on the horizontal axis. Typical units of wavenumber used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers (formerly called "microns"), symbol μm, which are related to the wavenumber in a reciprocal way. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electromagnetic Spectrum
The electromagnetic spectrum is the range of frequencies (the spectrum) of electromagnetic radiation and their respective wavelengths and photon energies. The electromagnetic spectrum covers electromagnetic waves with frequencies ranging from below one hertz to above 1025 hertz, corresponding to wavelengths from thousands of kilometers down to a fraction of the size of an atomic nucleus. This frequency range is divided into separate bands, and the electromagnetic waves within each frequency band are called by different names; beginning at the low frequency (long wavelength) end of the spectrum these are: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays at the high-frequency (short wavelength) end. The electromagnetic waves in each of these bands have different characteristics, such as how they are produced, how they interact with matter, and their practical applications. There is no known limit for long and short wavelengths. Extreme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Vibration
A molecular vibration is a periodic motion of the atoms of a molecule relative to each other, such that the center of mass of the molecule remains unchanged. The typical vibrational frequencies range from less than 1013 Hz to approximately 1014 Hz, corresponding to wavenumbers of approximately 300 to 3000 cm−1 and wavelengths of approximately 30 to 3 µm. For a diatomic molecule A−B, the vibrational frequency in s−1 is given by \nu = \frac \sqrt , where k is the force constant in dyne/cm or erg/cm2 and μ is the reduced mass given by \frac = \frac+\frac. The vibrational wavenumber in cm−1 is \tilde \;= \frac \sqrt, where c is the speed of light in cm/s. Vibrations of polyatomic molecules are described in terms of normal modes, which are independent of each other, but each normal mode involves simultaneous vibrations of different parts of the molecule. In general, a non-linear molecule with ''N'' atoms has 3''N'' – 6 normal modes of vibration, but a ''lin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopologue
In chemistry, isotopologues are molecules that differ only in their isotopic composition. They have the same chemical formula and bonding arrangement of atoms, but at least one atom has a different number of neutrons than the parent. An example is water, whose hydrogen-related isotopologues are: "light water" (HOH or ), "semi-heavy water" with the deuterium isotope in equal proportion to protium (HDO or ), " heavy water" with two deuterium isotopes of hydrogen per molecule ( or ), and "super-heavy water" or tritiated water ( or , as well as and , where some or all of the hydrogen atoms are replaced with the radioactive tritium isotope). Oxygen-related isotopologues of water include the commonly available form of heavy-oxygen water () and the more difficult to separate version with the isotope. Both elements may be replaced by isotopes, for example in the doubly labeled water isotopologue . All taken together, there are 9 different stable water isotopologues, and 9 radioacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome C Oxidase
The enzyme cytochrome c oxidase or Complex IV, (was , now reclassified as a translocasEC 7.1.1.9 is a large transmembrane protein complex found in bacteria, archaea, and mitochondria of eukaryotes. It is the last enzyme in the respiratory electron transport chain of cells located in the membrane. It receives an electron from each of four cytochrome c molecules and transfers them to one oxygen molecule and four protons, producing two molecules of water. In addition to binding the four protons from the inner aqueous phase, it transports another four protons across the membrane, increasing the transmembrane difference of proton electrochemical potential, which the ATP synthase then uses to synthesize ATP. Structure The complex The complex is a large integral membrane protein composed of several metal prosthetic sites and 14 protein subunits in mammals. In mammals, eleven subunits are nuclear in origin, and three are synthesized in the mitochondria. The complex contains ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter waves and acoustic waves can also be considered forms of radiative energy, and recently gravitational waves have been associated with a spectral signature in the context of the Laser Interferometer Gravitational-Wave Observatory (LIGO) In simpler terms, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum. Historically, spectroscopy originated as the study of the wavelength dependence of the absorption by gas phase matter of visible light dispersed by a prism. Spectroscopy, primarily in the electromagnetic spectrum, is a fundamental exploratory tool in the fields of astronomy, chemistry, materials science, and physics, allowing the composition, physical structure an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anti-Stokes Line
__NOTOC__ Stokes shift is the difference (in energy, wavenumber or frequency units) between positions of the band maxima of the absorption and emission spectra ( fluorescence and Raman being two examples) of the same electronic transition. It is named after Irish physicist George Gabriel Stokes. Sometimes Stokes shifts are given in wavelength units, but this is less meaningful than energy, wavenumber or frequency units because it depends on the absorption wavelength. For instance, a 50 nm Stokes shift from absorption at 300 nm is larger in terms of energy than a 50 nm Stokes shift from absorption at 600 nm. When a system (be it a molecule or atom) absorbs a photon, it gains energy and enters an excited state. One way for the system to relax is to emit a photon, thus losing its energy (another method would be the loss of energy as translational mode energy (via vibrational-translational or electronic-translational collisional processes with other atoms or molecules)). When ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inelastic Scattering
In chemistry, nuclear physics, and particle physics, inelastic scattering is a fundamental scattering process in which the kinetic energy of an incident particle is not conserved (in contrast to elastic scattering). In an inelastic scattering process, some of the energy of the incident particle is lost or increased. Although the term is historically related to the concept of inelastic collision in dynamics, the two concepts are quite distinct; inelastic collision in dynamics refers to processes in which the total macroscopic kinetic energy is not conserved. In general, scattering due to inelastic collisions will be inelastic, but, since elastic collisions often transfer kinetic energy between particles, scattering due to elastic collisions can also be ''in''elastic, as in Compton scattering meaning the two particles in the collision transfer energy causing a loss of energy in one particle. Electrons When an electron is the incident particle, the probability of inelastic scatter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectrum
A spectrum (plural ''spectra'' or ''spectrums'') is a condition that is not limited to a specific set of values but can vary, without gaps, across a continuum. The word was first used scientifically in optics to describe the rainbow of colors in visible light after passing through a prism. As scientific understanding of light advanced, it came to apply to the entire electromagnetic spectrum. It thereby became a mapping of a range of magnitudes (wavelengths) to a range of qualities, which are the perceived "colors of the rainbow" and other properties which correspond to wavelengths that lie outside of the visible light spectrum. Spectrum has since been applied by analogy to topics outside optics. Thus, one might talk about the " spectrum of political opinion", or the "spectrum of activity" of a drug, or the " autism spectrum". In these uses, values within a spectrum may not be associated with precisely quantifiable numbers or definitions. Such uses imply a broad range of co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around 1 millimeter (300 GHz) to the nominal red edge of the visible spectrum, around 700 nanometers (430 THz). Longer IR wavelengths (30 μm-100 μm) are sometimes included as part of the terahertz radiation range. Almost all black-body radiation from objects near room temperature is at infrared wavelengths. As a form of electromagnetic radiation, IR propagates energy and momentum, exerts radiation pressure, and has properties corresponding to both those of a wave and of a particle, the photon. It was long known that fires emit invisible heat; in 1681 the pioneering experimenter Edme Mariotte showed that glass, though transparent to sunlight, obstructed radiant heat. In 1800 the astronomer Sir William Herschel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |