|
Reichardt's Dye
Reichardt's dye (Betaine 30) is an organic dye belonging to the class of azo merocyanine betaines. This dye is notable for its solvatochromic properties, meaning it changes color depending on the solvent in which it is dissolved. It has one of the largest solvatochromic effects ever observed, with color varying across the entire visible spectrum. As a result, it gives striking visual results for chemical demonstrations. This chemical is named for , who developed it when working as a doctoral student in the lab of . It is thus also sometimes called Dimroth–Reichardt dye. The names also sometimes refer to some close chemical analogs, in particular, the one having para substituted ''tert''-butyl groups on the phenyl rings. Synthesis A newer synthesis is: 2,6-Diphenylphenol is nitrated with diluted nitric acid to 4-nitro-2,6-diphenylphenol and subsequently reduced with sodium dithionite to the amine. This is reacted in presence of sodium acetate in ethanol with 2,4,6-t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitration
In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group () into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters () between Alcohol (chemistry), alcohols and nitric acid (as occurs in the Organic synthesis, synthesis of nitroglycerin). The difference between the resulting molecular structures of nitro compounds and nitrates () is that the nitrogen atom in nitro compounds is directly Chemical bond, bonded to a non-oxygen atom (typically carbon or another nitrogen atom), whereas in nitrate esters (also called organic nitrates), the nitrogen is bonded to an oxygen atom that in turn usually is bonded to a carbon atom (nitrito group). There are many major industrial applications of nitration in the strict sense; the most important by volume are for the production of nitroaromatic compounds such as nitrobenzene. The technology is long-standing and mature. : Nitration rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
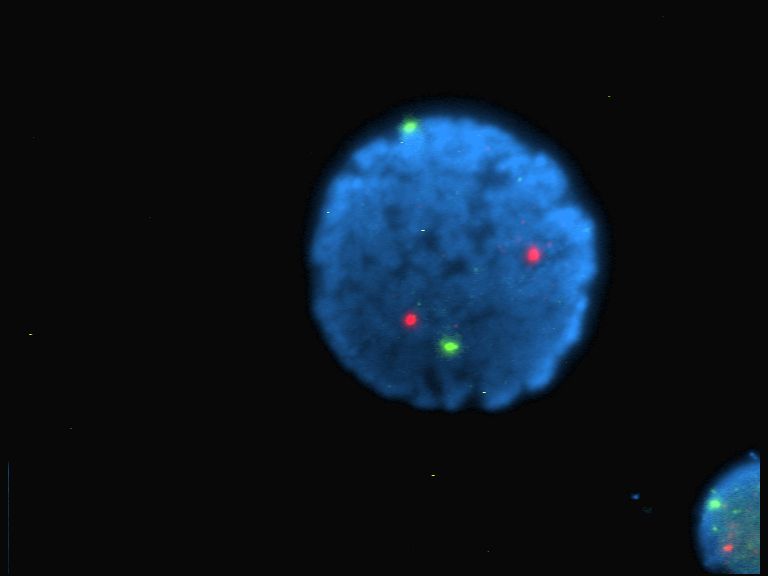
Fluorescent Dyes
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds. Fluorophores are sometimes used alone, as a tracer in fluids, as a dye for staining of certain structures, as a substrate of enzymes, or as a probe or indicator (when its fluorescence is affected by environmental aspects such as polarity or ions). More generally they are covalently bonded to macromolecules, serving as a markers (or dyes, or tags, or reporters) for affine or bioactive reagents (antibodies, peptides, nucleic acids). Fluorophores are notably used to stain tissues, cells, or materials in a variety of analytical methods, such as fluorescent imaging and spectroscopy. Fluorescein, via its amine-reactive isothiocyanate derivative fluorescein isothiocyanate (FITC), has been one of the most popular fluorophores. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions . Sodium hydroxide is a highly corrosive base (chemistry), base and alkali that decomposes lipids and proteins at ambient temperatures and at high concentrations may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates . The monohydrate crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students. Sodium hydroxide is used in many industries: in the making of wood pulp and paper, tex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
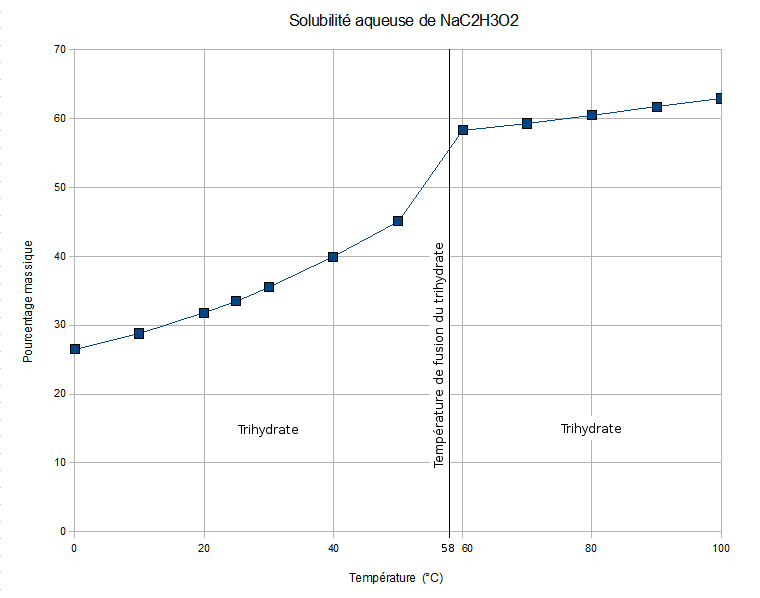
Sodium Acetate
Sodium acetate, CH3COONa, also abbreviated Sodium, NaOxygen, OAcetyl, Ac, is the sodium Salt (chemistry), salt of acetic acid. This salt is colorless, deliquescent, and hygroscopy, hygroscopic. Applications Biotechnological Sodium acetate is used as the carbon source (biology) , carbon source for culturing Bacterial growth, bacteria. Sodium acetate can also be useful for increasing yields of DNA extraction, DNA isolation by ethanol precipitation. Industrial Sodium acetate is used in the textile industry to neutralize sulfuric acid waste streams and also as a photoresist while using aniline dyes. It is also a pickling (metal), pickling agent in chrome Tanning (leather), tanning and helps to impede vulcanization of chloroprene in synthetic rubber production. It is also used to reduce static electricity during production of disposable cotton pads. Concrete longevity Sodium acetate is used as a sealant to mitigate water damage to concrete. It is environm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Dithionite
Sodium dithionite (also known as sodium hydrosulfite) is a white crystalline powder with a sulfurous odor. Although it is stable in dry air, it decomposes in hot water and in acid solutions. Structure left, 220px, Packing of sodium dithionite ions in a crystal, showing the saw-horse geometry of the dianion. Color code: red = O, yellow = S. The structure has been examined by Raman spectroscopy and X-ray crystallography. The dithionite dianion has C symmetry, with almost eclipsed with a 16° O-S-S-O torsional angle. In the dihydrated form (), the dithionite anion has gauche 56° O-S-S-O torsional angle. A weak S-S bond is indicated by the S-S distance of 239 pm, which is elongated by ca. 30 pm relative to a typical S-S bond. Because this bond is fragile, the dithionite anion dissociates in solution into the O2sup>− radicals, as has been confirmed by EPR spectroscopy. It is also observed that 35S undergoes rapid exchange between S2O42− and SO2 in neutral or acidic solu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% , it is referred to as ''fuming nitric acid''. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%. Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as synthetic dyes and medicines (e.g. metronidazole). Nitric acid is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |