|
Ramucirumab
Ramucirumab (LY3009806, IMC-1121B, trade name Cyramza) is a fully human monoclonal antibody (IgG1) developed for the treatment of solid tumors. This drug was developed by ImClone Systems Inc. It was isolated from a native phage display library from Dyax. Approved uses On 21 April 2014, the US Food and Drug Administration (FDA) approved ramucirumab as a single-agent treatment for advanced gastric cancer or gastro-esophageal junction (GEJ) adenocarcinoma after prior treatment with fluoropyrimidine- or platinum-containing chemotherapy. The approval was based on the results of the REGARD trial, a phase III, international, randomized, double-blind, placebo-controlled study, that evaluated the safety and efficacy of ramucirumab combinated with best supportive care versus placebo. This trial has been criticised for its use of a placebo control arm, which does not reflect standard of care in most Western countries. Ramucirumab has also been studied in combination with paclitaxel (a t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ImClone Systems
ImClone Systems Incorporated was a biopharmaceutical company dedicated to developing biologic medicines in the area of oncology. It was founded in 1984 and had its corporate headquarters in Bridgewater, New Jersey, and its research headquarters in New York City. On October 6, 2008, it accepted a $6.5 billion acquisition offer from Eli Lilly and Company, and became a fully-owned subsidiary of Eli Lilly and Company on November 24, 2008. Prior to the acquisition, it was traded on the NASDAQ stock exchange under the symbol IMCL. Imclone lost its separate identity in 2014 when its former ImClone research and manufacturing sites were renamed ''Eli Lilly and Company''. Insider trading scandal ImClone's stock price dropped sharply at the end of 2001 when its drug Erbitux, an experimental monoclonal antibody, failed to get the expected Food and Drug Administration (FDA) approval. It was later revealed by the U.S. Securities and Exchange Commission that numerous executives sold their sto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
VEGFR2
Kinase insert domain receptor (KDR, a type IV receptor tyrosine kinase) also known as vascular endothelial growth factor receptor 2 (VEGFR-2) is a VEGF receptor. ''KDR'' is the human gene encoding it. KDR has also been designated as CD309 (cluster of differentiation 309). KDR is also known as Flk1 (Fetal Liver Kinase 1). The Q472H germline ''KDR'' genetic variant affects VEGFR-2 phosphorylation and has been found to associate with microvessel density in NSCLC. Interactions Kinase insert domain receptor has been shown to interact with SHC2, Annexin A5 and SHC1. See also * Cluster of differentiation * VEGF receptors VEGF receptors are receptors for vascular endothelial growth factor (VEGF). There are three main subtypes of VEGFR, numbered 1, 2 and 3. Also, they may be membrane-bound (mbVEGFR) or soluble (sVEGFR), depending on alternative splicing. Inhi ... References Further reading * * * * * * * * External links * * Clusters of diff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoropyrimidine
Fluoropyrimidines are a class of anti-cancer drugs, or more specifically antimetabolites, and include: * Capecitabine * Carmofur (HCFU) * Doxifluridine * Fluorouracil (5-FU) * Tegafur Tegafur is a chemotherapeutic prodrug of 5-fluorouracil (5-FU) used in the treatment of cancers. It is a component of the combination drug tegafur/uracil. When metabolised, it becomes 5-FU. It was patented in 1967 and approved for medical use in ... {{chemistry index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would not onl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
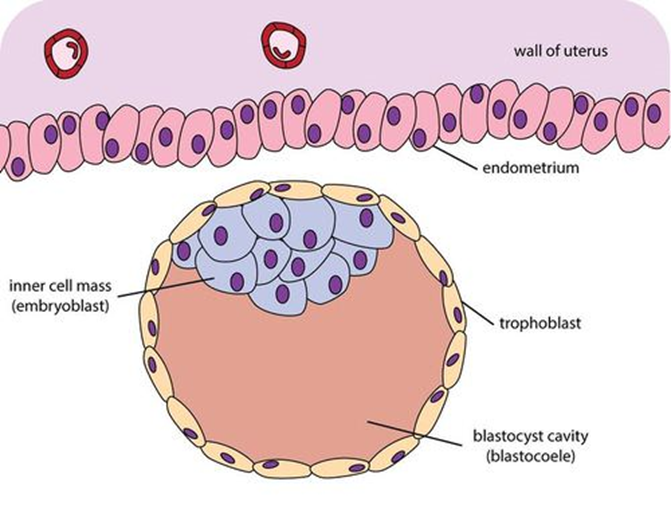
Cavitation (biology)
Cavitation is a process in early embryonic development that follows cleavage. Cavitation is the formation of the blastocoel, a fluid-filled cavity that defines the blastula, or in mammals the blastocyst. After fertilization, cell division of the zygote occurs which results in the formation of a solid ball of cells (blastomeres) called the morula. Further division of cells increases their number in the morula, and the morula differentiates them into two groups. The internal cells become the inner cell mass, and the outer cells become the trophoblast. Before cell differentiation takes place there are two transcription factors, Oct-4 and nanog that are uniformly expressed on all of the cells, but both of these transcription factors are turned off in the trophoblast once it has formed. The trophoblast cells form tight junctions between them making the structure leakproof. Trophoblast cells have sodium pumps on their membranes, and pump sodium into the centre of the morula. This dr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Overall Survival
Survival rate is a part of survival analysis. It is the proportion of people in a study or treatment group still alive at a given period of time after diagnosis. It is a method of describing prognosis in certain disease conditions, and can be used for the assessment of standards of therapy. The survival period is usually reckoned from date of diagnosis or start of treatment. Survival rates are based on the population as a whole and cannot be applied directly to an individual. There are various types of survival rates (discussed below). They often serve as endpoints of clinical trials and should not be confused with mortality rates, a population metric. Overall survival Patients with a certain disease (for example, colorectal cancer) can die directly from that disease or from an unrelated cause (for example, a car accident). When the precise cause of death is not specified, this is called the overall survival rate or observed survival rate. Doctors often use mean overall survival ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Placebo-controlled Study
Placebo-controlled studies are a way of testing a medical therapy in which, in addition to a group of subjects that receives the treatment to be evaluated, a separate control group receives a sham "placebo" treatment which is specifically designed to have no real effect. Placebos are most commonly used in blinded trials, where subjects do not know whether they are receiving real or placebo treatment. Often, there is also a further "natural history" group that does not receive any treatment at all. The purpose of the placebo group is to account for the placebo effect, that is, effects from treatment that do not depend on the treatment itself. Such factors include knowing one is receiving a treatment, attention from health care professionals, and the expectations of a treatment's effectiveness by those running the research study. Without a placebo group to compare against, it is not possible to know whether the treatment itself had any effect. Patients frequently show improvement e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double-blind
In a blind or blinded experiment, information which may influence the participants of the experiment is withheld until after the experiment is complete. Good blinding can reduce or eliminate experimental biases that arise from a participants' expectations, observer's effect on the participants, observer bias, confirmation bias, and other sources. A blind can be imposed on any participant of an experiment, including subjects, researchers, technicians, data analysts, and evaluators. In some cases, while blinding would be useful, it is impossible or unethical. For example, it is not possible to blind a patient to their treatment in a physical therapy intervention. A good clinical protocol ensures that blinding is as effective as possible within ethical and practical constraints. During the course of an experiment, a participant becomes unblinded if they deduce or otherwise obtain information that has been masked to them. For example, a patient who experiences a side effect may correc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Randomized Controlled Trial
A randomized controlled trial (or randomized control trial; RCT) is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trials that compare the effects of drugs, surgical techniques, medical devices, diagnostic procedures or other medical treatments. Participants who enroll in RCTs differ from one another in known and unknown ways that can influence study outcomes, and yet cannot be directly controlled. By Random assignment, randomly allocating participants among compared treatments, an RCT enables ''statistical control'' over these influences. Provided it is designed well, conducted properly, and enrolls enough participants, an RCT may achieve sufficient control over these confounding factors to deliver a useful comparison of the treatments studied. Definition and examples An RCT in clinical research typically compares a proposed new treatment against an existing Standard of care#Medical standard of care, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sorafenib
Sorafenib, sold under the brand name Nexavar, is a kinase inhibitor drug approved for the treatment of primary kidney cancer (advanced renal cell carcinoma), advanced primary liver cancer ( hepatocellular carcinoma), FLT3-ITD positive AML and radioactive iodine resistant advanced thyroid carcinoma. Mechanism of action Sorafenib is a protein kinase inhibitor with activity against many protein kinases, including VEGFR, PDGFR and RAF kinases. Of the RAF kinases, sorafenib is more selective for c-Raf than B-RAF. (See BRAF (gene)#Sorafenib for details the drug's interaction with B-Raf.) Sorafenib treatment induces autophagy, which may suppress tumor growth. Based on its 1,3-disubstituted urea structure, sorafenib is also a potent soluble epoxide hydrolase inhibitor and this activity likely reduces the severity of its adverse effects. Medical uses Sorafenib is indicated as a treatment for advanced renal cell carcinoma (RCC), unresectable hepatocellular carcinomas (HCC) and thy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-fetoprotein
Alpha-fetoprotein (AFP, α-fetoprotein; also sometimes called alpha-1-fetoprotein, alpha-fetoglobulin, or alpha fetal protein) is a protein that in humans is encoded by the ''AFP'' gene. The ''AFP'' gene is located on the ''q'' arm of chromosome 4 (4q25). Maternal AFP serum level is used to screen for Down syndrome, neural tube defects, and other chromosomal abnormalities. AFP is a major plasma protein produced by the yolk sac and the fetal liver during fetal development. It is thought to be the fetal analog of serum albumin. AFP binds to copper, nickel, fatty acids and bilirubin and is found in monomeric, dimeric and trimeric forms. Structure AFP is a glycoprotein of 591 amino acids and a carbohydrate moiety. Function The function of AFP in adult humans is unknown. AFP is the most abundant plasma protein found in the human fetus. Maternal plasma levels peak near the end of the first trimester, and begin decreasing prenatally at that time, then decrease rapidly after birt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer in adults and is currently the most common cause of death in people with cirrhosis. HCC is the third leading cause of cancer-related deaths worldwide. It occurs in the setting of chronic liver inflammation, and is most closely linked to chronic viral hepatitis infection (hepatitis B or C) or exposure to toxins such as alcohol, aflatoxin, or pyrrolizidine alkaloids. Certain diseases, such as hemochromatosis and alpha 1-antitrypsin deficiency, markedly increase the risk of developing HCC. Metabolic syndrome and NASH are also increasingly recognized as risk factors for HCC. As with any cancer, the treatment and prognosis of HCC vary depending on the specifics of tumor histology, size, how far the cancer has spread, and overall health. The vast majority of HCC cases and the lowest survival rates after treatment occur in Asia and sub-Saharan Africa, in countries where hepatitis B infection is endem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |