|
Raf Kinase Inhibitor Protein
The Raf kinase inhibitor protein (RKIP) is a kinase inhibitor protein, that regulates many signaling pathways within the cell. RKIP is a member of the phosphatidylethanolamine-binding protein family and has displayed disruptive regulation on the Raf-1- MEK1/2, ERK1/2 and NF-kappaB signalling pathways, by interaction with the Raf-1 kinase. RKIP has also been shown to inhibit G protein coupled receptor kinases (GRK) when phosphorylated by protein kinase C.Lorenz K, Lohse MJ, Quitterer U (2003): ''Protein kinase C switches the Raf kinase inhibitor from Raf-1 to GRK-2.'' Nature 426: 574-579. Via this mechanism it has been shown to exert beneficial effects on cardiac structure and function.Schmid E, Neef S, Berlin C, Tomasovic A, Kahlert K, Nordbeck P, Deiss K, Denzinger S, Herrmann S, Wettwer E, Weidendorfer M, Becker D, Schäfer F, Wagner N, Ergün S, Schmitt JP, Katus HA, Weidemann F, Ravens U, Maack C, Hein L, Ertl G, Müller OJ, Maier LS, Lohse MJ, Lorenz K (2015): ''Cardiac RKIP in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-Raf
RAF proto-oncogene serine/threonine-protein kinase, also known as proto-oncogene c-RAF or simply c-Raf or even Raf-1, is an enzyme that in humans is encoded by the ''RAF1'' gene. The c-Raf protein is part of the ERK1/2 pathway as a MAP kinase (MAP3K) that functions downstream of the Ras subfamily of membrane associated GTPases. C-Raf is a member of the Raf kinase family of serine/threonine-specific protein kinases, from the TKL (Tyrosine-kinase-like) group of kinases. Discovery The first Raf gene, v-Raf was found in 1983. It was isolated from the murine retrovirus bearing the number 3611. It was soon demonstrated to be capable to transform rodent fibroblasts to cancerous cell lines, so this gene was given the name Virus-induced Rapidly Accelerated Fibrosarcoma (V-RAF). A year later, another transforming gene was found in the avian retrovirus MH2, named v-Mil - that turned out to be highly similar to v-Raf. Researchers were able to demonstrate that these genes encode enzymes th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Kinase Inhibitor
A protein kinase inhibitor is a type of enzyme inhibitor that blocks the action of one or more protein kinases. Protein kinases are enzymes that phosphorylate (add a phosphate, or PO4, group) to a protein and can modulate its function. The phosphate groups are usually added to serine, threonine, or tyrosine amino acids on the protein: most kinases act on both serine and threonine, the tyrosine kinases act on tyrosine, and a number (dual-specificity kinases) act on all three. There are also protein kinases that phosphorylate other amino acids, including histidine kinases that phosphorylate histidine residues. Phosphorylation regulates many biological processes, and protein kinase inhibitors can be used to treat diseases due to hyperactive protein kinases (including mutant or overexpressed kinases in cancer) or to modulate cell functions to overcome other disease drivers. Clinical use Kinase inhibitors such as dasatinib are often used in the treatment of cancer and inflammation. So ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
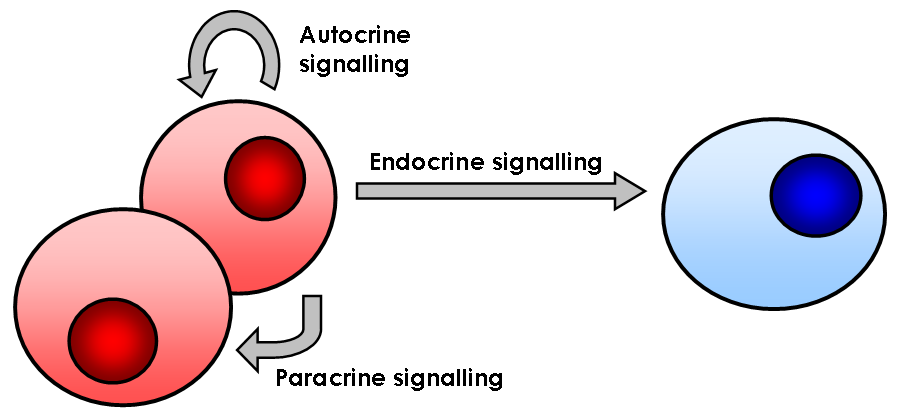
Signaling Pathway
In biology, cell signaling (cell signalling in British English) or cell communication is the ability of a cell to receive, process, and transmit signals with its environment and with itself. Cell signaling is a fundamental property of all cellular life in prokaryotes and eukaryotes. Signals that originate from outside a cell (or extracellular signals) can be physical agents like mechanical pressure, voltage, temperature, light, or chemical signals (e.g., small molecules, peptides, or gas). Cell signaling can occur over short or long distances, and as a result can be classified as autocrine, juxtacrine, intracrine, paracrine, or endocrine. Signaling molecules can be synthesized from various biosynthetic pathways and released through passive or active transports, or even from cell damage. Receptors play a key role in cell signaling as they are able to detect chemical signals or physical stimuli. Receptors are generally proteins located on the cell surface or within the interio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mitogen-activated Protein Kinase Kinase
Mitogen-activated protein kinase kinase (also known as MAP2K, MEK, MAPKK) is a dual-specificity kinase enzyme which phosphorylates mitogen-activated protein kinase (MAPK). MAP2K is classified as . There are seven genes: * (a.k.a. MEK1) * (a.k.a. MEK2) * (a.k.a. MKK3) * (a.k.a. MKK4) * (a.k.a. MKK5) * (a.k.a. MKK6) * (a.k.a. MKK7) The activators of p38 (MKK3 and MKK6), JNK (MKK4 and MKK7), and ERK (MEK1 and MEK2) define independent MAP kinase signal transduction pathways. The acronym MEK derives from MAPK/ERK Kinase. Role in melanoma MEK is a member of the MAPK signaling cascade that is activated in melanoma. When MEK is inhibited, cell proliferation is blocked and apoptosis (controlled cell death) is induced. See also * Signal transduction * MAP kinase * MAP kinase kinase kinase * MAP kinase kinase kinase kinase Mitogen-activated protein kinase kinase kinase kinase (MAP4K) is a family of proteins involved in cellular signal transduction. * MAP4K1 (aka HPK1) * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MAPK/ERK Pathway
The MAPK/ERK pathway (also known as the Ras-Raf-MEK-ERK pathway) is a chain of proteins in the cell that communicates a signal from a receptor on the surface of the cell to the DNA in the nucleus of the cell. The signal starts when a signaling molecule binds to the receptor on the cell surface and ends when the DNA in the nucleus expresses a protein and produces some change in the cell, such as cell division. The pathway includes many proteins, such as mitogen-activated protein kinases (MAPKs), originally called extracellular signal-regulated kinases (ERKs), which communicate by adding phosphate groups to a neighboring protein ( phosphorylating it), thereby acting as an "on" or "off" switch. When one of the proteins in the pathway is mutated, it can become stuck in the "on" or "off" position, a necessary step in the development of many cancers. In fact, components of the MAPK/ERK pathway were first discovered in cancer cells, and drugs that reverse the "on" or "off" switch are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Kinase C
In cell biology, Protein kinase C, commonly abbreviated to PKC (EC 2.7.11.13), is a family of protein kinase enzymes that are involved in controlling the function of other proteins through the phosphorylation of hydroxyl groups of serine and threonine amino acid residues on these proteins, or a member of this family. PKC enzymes in turn are activated by signals such as increases in the concentration of diacylglycerol (DAG) or calcium ions (Ca2+). Hence PKC enzymes play important roles in several signal transduction cascades. In biochemistry, the PKC family consists of fifteen isozymes in humans. They are divided into three subfamilies, based on their second messenger requirements: conventional (or classical), novel, and atypical. Conventional (c)PKCs contain the isoforms α, βI, βII, and γ. These require Ca2+, DAG, and a phospholipid such as phosphatidylserine for activation. Novel (n)PKCs include the δ, ε, η, and θ isoforms, and require DAG, but do not require Ca2+ for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nature (journal)
''Nature'' is a British weekly scientific journal founded and based in London, England. As a multidisciplinary publication, ''Nature'' features peer-reviewed research from a variety of academic disciplines, mainly in science and technology. It has core editorial offices across the United States, continental Europe, and Asia under the international scientific publishing company Springer Nature. ''Nature'' was one of the world's most cited scientific journals by the Science Edition of the 2019 ''Journal Citation Reports'' (with an ascribed impact factor of 42.778), making it one of the world's most-read and most prestigious academic journals. , it claimed an online readership of about three million unique readers per month. Founded in autumn 1869, ''Nature'' was first circulated by Norman Lockyer and Alexander Macmillan as a public forum for scientific innovations. The mid-20th century facilitated an editorial expansion for the journal; ''Nature'' redoubled its efforts in exp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nature Medicine
''Nature Medicine'' is a monthly Peer review, peer-reviewed medical journal published by Nature Portfolio covering all aspects of medicine. It was established in 1995. The journal seeks to publish research papers that "demonstrate novel insight into disease processes, with direct evidence of the physiological relevance of the results". As with other ''Nature'' journals, there is no external editorial board, with editorial decisions being made by an in-house team, although peer review by external expert referees forms a part of the review process. The editor-in-chief is João Monteiro (editor), João Monteiro. According to the ''Journal Citation Reports'', the journal has a 2021 impact factor of 87.241, ranking it 1st out of 296 journals in the category "Biochemistry & Molecular Biology". References External links * {{Portal bar, Medicine Publications established in 1995 Nature Research academic journals General medical journals Monthly journals English-language journa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |