|
Polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer, monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many forms of polymerization and different systems exist to categorize them. In chemical compounds, polymerization can occur via a variety of reaction mechanisms that vary in complexity due to the functional groups present in the reactants and their inherent steric effects. In more straightforward polymerizations, alkenes form polymers through relatively simple free-radical reaction, radical reactions; in contrast, reactions involving substitution at a carbonyl group require more complex synthesis due to the way in which reactants polymerize. Alkanes can also be polymerized, but only with the help of strong acids. As alkenes can polymerize in somewhat straightforward radical reactions, they form useful compounds such as polyethylene and p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copolymer
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are sometimes called ''bipolymers''. Those obtained from three and four monomers are called ''terpolymers'' and ''quaterpolymers'', respectively. Copolymers can be characterized by a variety of techniques such as NMR spectroscopy and size-exclusion chromatography to determine the molecular size, weight, properties, and composition of the material. Commercial copolymers include acrylonitrile butadiene styrene (ABS), styrene/butadiene co-polymer (SBR), nitrile rubber, styrene-acrylonitrile, styrene-isoprene-styrene (SIS) and ethylene-vinyl acetate, all of which are formed by chain-growth polymerization. Another production mechanism is step-growth polymerization, which is used to produce the nylon-12/6/66 copolymer of nylon 12, nylon 6 and nylon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
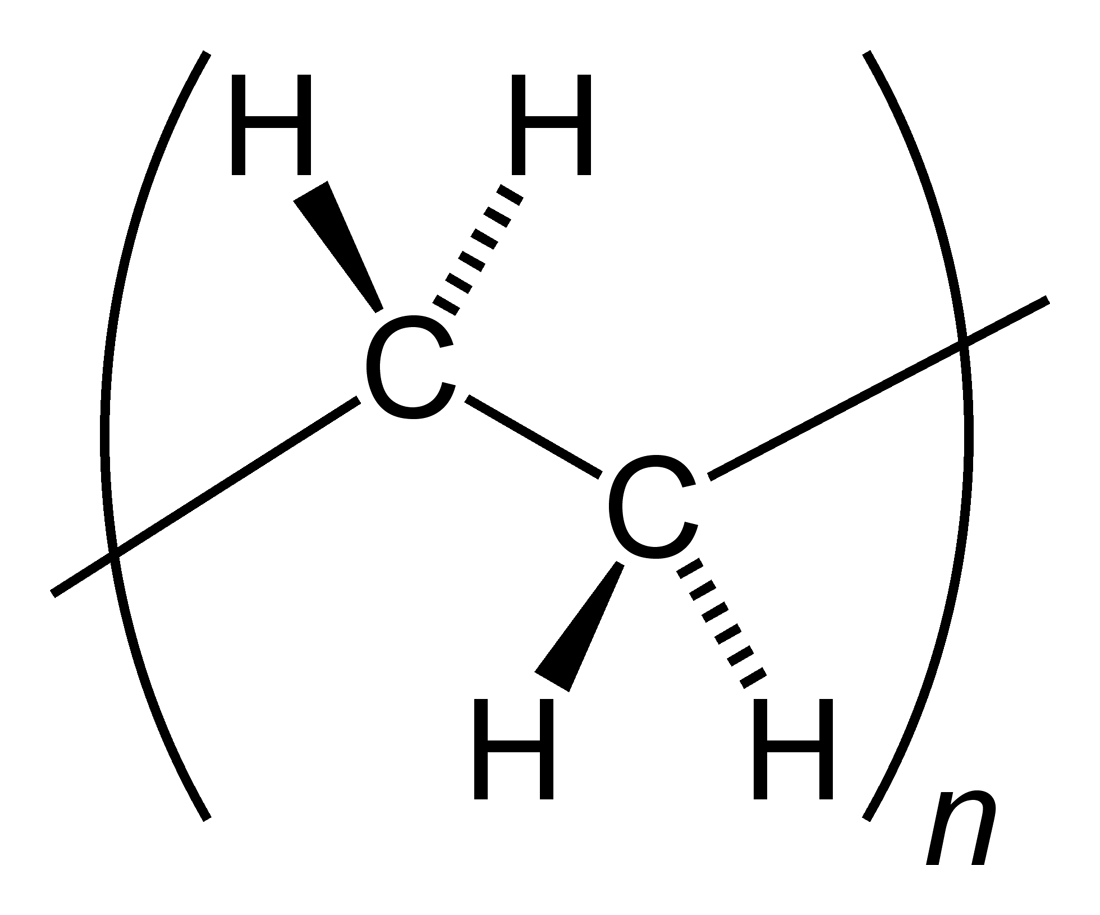
Polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Condensation Polymer
In polymer chemistry, condensation polymers are any kind of polymers whose process of polymerization involves a condensation reaction (i.e. a small molecule, such as water or methanol, is produced as a byproduct). Condensation polymers are formed by polycondensation, when the polymer is formed by condensation reactions between species of all degrees of polymerization, or by condensative chain polymerization, when the polymer is formed by sequential addition of monomers to an active site in a chain reaction. The main alternative forms of polymerization are chain polymerization and polyaddition, both of which give addition polymers. Condensation polymerization is a form of step-growth polymerization. Linear polymers are produced from bifunctional monomers, i.e. compounds with two reactive end-groups. Common condensation polymers include polyamides, polyacetals, and proteins. Polyamides One important class of condensation polymers are polyamides. They arise from the reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging ( plastic bags, plastic films, geomembranes and containers including bottles, etc.). , over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market. Many kinds of polyethylene are known, with most having the chemical formula (C2H4)''n''. PE is usually a mixture of similar polymers of ethylene, with various values of ''n''. It can be ''low-density'' or ''high-density'': low-density polyethylene is extruded using high pressure () and high temperature (), while high-density polyethylene is extruded using low pressure () and low temperature (). Polyethylene is usually thermoplastic, but it can be modified to become thermosetting instead, for example, in cross-linked polyethylene. History Polyethylene was first synthesized by the German chemist Hans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polystyrene Formation
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It is a poor barrier to oxygen and water vapour and has a relatively low melting point. Polystyrene is one of the most widely used plastics, the scale of its production being several million tonnes per year. Polystyrene can be naturally transparent, but can be colored with colorants. Uses include protective packaging (such as packing peanuts and in the jewel cases used for storage of optical discs such as CDs and occasionally DVDs), containers, lids, bottles, trays, tumblers, disposable cutlery, in the making of models, and as an alternative material for phonograph records. As a thermoplastic polymer, polystyrene is in a solid (glassy) state at room temperature but flows if heated above about 100 °C, its glass transition temperature. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Autoacceleration
Autoacceleration (gel effect, Trommsdorff–Norrish effect) is a dangerous reaction behavior that can occur in free-radical polymerization systems. It is due to the localized increases in viscosity of the polymerizing system that slow termination reactions. The removal of reaction obstacles therefore causes a rapid increase in the overall rate of reaction, leading to possible reaction runaway and altering the characteristics of the polymers produced.Alger, Mark. Polymer Science Dictionary. New York: Elsevier Applied Science, 1989. 28. Background Autoacceleration of the overall rate of a free-radical polymerization system has been noted in many bulk polymerization systems. The polymerization of methyl methacrylate, for example, deviates strongly from classical mechanism behavior around 20% conversion; in this region the conversion and molecular mass of the polymer produced increases rapidly. This increase of polymerization is usually accompanied by a large rise in temperature if ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization. Classification Monomers can be classified in many ways. They can be subdivided into two broad classes, depending on the kind of the polymer that they form. Monomers that participate in condensation polymerization have a different stoichiometry than monomers that participate in addition polymerization: : Other classifications include: *natural vs synthetic monomers, e.g. glycine vs caprolactam, respectively *polar vs nonpolar monomers, e.g. vinyl acetate vs ethylene, respectively *cyclic vs linear, e.g. ethylene oxide vs ethylene glycol, respectively The polymerization of one kind of monomer gives a homopolymer. Many polymers are copolymers, meaning that they are derived from two different monomers. In the case of condensation polymerizations, the r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyester
Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing. Polyester fibers are sometimes spun together with natural fibers to produce a cloth with blended properties. Cotton-polyester blends can be strong, wrinkle- and tear-resistant, and reduce shrinking. Synthetic fibers using polyester have high water, wind and environmental resistance compared to plant-derived fibers. They are less Fireproofing, fire-resistant and can melt when ignited. Liquid crystalline polyesters are among the first industrially used liquid crystal polymers. They are use ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molar Mass Distribution
The molar mass distribution (or molecular weight distribution) describes the relationship between the number of moles of each polymer species (Ni) and the molar mass (Mi) of that species. In linear polymers, the individual polymer chains rarely have exactly the same degree of polymerization and molar mass, and there is always a distribution around an average value. The molar mass distribution of a polymer may be modified by polymer fractionation. Definitions of molar mass average Different average values can be defined, depending on the statistical method applied. In practice, four averages are used, representing the weighted mean taken with the mole fraction, the weight fraction, and two other functions which can be related to measured quantities: *''Number average molar mass'' (Mn), also loosely referred to as ''number average molecular weight'' (NAMW). *''Mass average molar mass'' (Mw), where ''w'' stands for weight; also commonly referred to as ''weight average'' or ''weight ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkenes
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (chemistry), products, which usually have properties different from the reactants. Reactions often consist of a sequence o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclization
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon (i.e., are carbocycles), none of the atoms are carbon (inorganic cyclic compounds), or where both carbon and non-carbon atoms are present (heterocyclic compounds). Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size (e.g., < 17 total atoms) numbers in the many b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |