|
Polyimide
Polyimide (sometimes abbreviated PI) is a polymer containing imide groups belonging to the class of high-performance plastics. With their high heat-resistance, polyimides enjoy diverse applications in roles demanding rugged organic materials, e.g. high temperature fuel cells, displays, and various military roles. A classic polyimide is Kapton, which is produced by condensation of pyromellitic dianhydride and 4,4'-oxydianiline.Wright, Walter W. and Hallden-Abberton, Michael (2002) "Polyimides" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim. History The first polyimide was discovered in 1908 by Bogart and Renshaw. They found that 4-amino phthalic anhydride does not melt when heated but does release water upon the formation of a high molecular weight polyimide. The first semialiphatic polyimide was prepared by Edward and Robinson by melt fusion of diamines and tetra acids or diamines and diacids / diester. However, the first polyimide of significant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyimide Formation (schematic) V1
Polyimide (sometimes abbreviated PI) is a polymer containing imide groups belonging to the class of high-performance plastics. With their high heat-resistance, polyimides enjoy diverse applications in roles demanding rugged organic materials, e.g. high temperature fuel cells, displays, and various military roles. A classic polyimide is Kapton, which is produced by condensation of pyromellitic dianhydride and 4,4'-Oxydianiline, 4,4'-oxydianiline.Wright, Walter W. and Hallden-Abberton, Michael (2002) "Polyimides" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim. History The first polyimide was discovered in 1908 by Bogart and Renshaw. They found that 4-amino phthalic anhydride does not melt when heated but does release water upon the formation of a high molecular weight polyimide. The first semialiphatic polyimide was prepared by Edward and Robinson by melt fusion of diamines and tetra acids or diamines and diacids / diester. However, the first polyimide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyimide
Polyimide (sometimes abbreviated PI) is a polymer containing imide groups belonging to the class of high-performance plastics. With their high heat-resistance, polyimides enjoy diverse applications in roles demanding rugged organic materials, e.g. high temperature fuel cells, displays, and various military roles. A classic polyimide is Kapton, which is produced by condensation of pyromellitic dianhydride and 4,4'-oxydianiline.Wright, Walter W. and Hallden-Abberton, Michael (2002) "Polyimides" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim. History The first polyimide was discovered in 1908 by Bogart and Renshaw. They found that 4-amino phthalic anhydride does not melt when heated but does release water upon the formation of a high molecular weight polyimide. The first semialiphatic polyimide was prepared by Edward and Robinson by melt fusion of diamines and tetra acids or diamines and diacids / diester. However, the first polyimide of significant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermoset Polymer Matrix
A thermoset polymer matrix is a synthetic polymer reinforcement where polymers act as binder or matrix to secure in place incorporated particulates, fibres or other reinforcements. They were first developed for structural applications, such as glass-reinforced plastic radar domes on aircraft and graphite-epoxy payload bay doors on the Space Shuttle. They were first used after World War II, and continuing research has led to an increased range of thermoset resins, polymers or plastics, as well as engineering grade thermoplastics. They were all developed for use in the manufacture of polymer composites with enhanced and longer-term service capabilities. Thermoset polymer matrix technologies also find use in a wide diversity of non-structural industrial applications. The foremost types of thermosetting polymers used in structural composites are benzoxazine resins, bis-maleimide resins (BMI), cyanate ester resins, epoxy (epoxide) resins, phenolic (PF) resins, unsaturated polyester ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kapton
Structure of poly-oxydiphenylene-pyromellitimide Kapton insulating pads for mounting electronic parts on a heat sink Kapton is a polyimide film used in flexible printed circuits (flexible electronics) and space blankets, which are used on spacecraft, satellites, and various space instruments. Invented by the DuPont Corporation in the 1960s, Kapton remains stable (in isolation) across a wide range of temperatures, from . History Kapton was invented by the DuPont Corporation in the 1960s. The name ''Kapton'' is a registered trademark of E. I. du Pont de Nemours and Company. Chemistry and variants Kapton synthesis is an example of the use of a dianhydride in step polymerization. The intermediate polymer, known as a ''poly(amic acid)'', is soluble because of strong hydrogen bonds to the polar solvents usually employed in the reaction. The ring closure is carried out at high temperatures of . The chemical name for Kapton K and HN is ''poly (4,4'-oxydiphenylene-pyromellit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imide
In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applications, imides are best known as components of high-strength polymers, called polyimides. Inorganic imides are also known as solid state or gaseous compounds, and the imido group (=NH) can also act as a ligand. Nomenclature Most imides are cyclic compounds derived from dicarboxylic acids, and their names reflect the parent acid. Examples are succinimide, derived from succinic acid, and phthalimide, derived from phthalic acid. For imides derived from amines (as opposed to ammonia), the ''N''-substituent is indicated by a prefix. For example, N-ethylsuccinimide is derived from succinic acid and ethylamine. Isoimides are isomeric with normal imides and have the formula RC(O)OC(NR′)R″. They are often intermediates that convert to the more symmet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyromellitic Dianhydride
Pyromellitic dianhydride (PMDA) is an organic compound with the formula C6H2(C2O3)2. It is the double carboxylic acid anhydride that is used in the preparation of polyimide polymers such as Kapton. It is a white, hygroscopic solid. It forms a hydrate. Preparation It is prepared by gas-phase oxidation of 1,2,4,5-tetramethylbenzene (or related tetrasubstituted benzene derivatives). An idealized equation is: :C6H2(CH3)4 + 6 O2 → C6H2(C2O3)2 + 6 H2O In the laboratory, it can be prepared by dehydration of pyromellitic acid using acetic anhydride. Reactions PMDA is an electron-acceptor, forming a variety of charge-transfer complexes. It reacts with amines to diimides, C6H2 CO)2NRsub>2 which also have acceptor properties. Safety Evidence suggests that PMDA causes occupational asthma Occupational asthma is new onset asthma or the recurrence of previously quiescent asthma directly caused by exposure to an agent at workplace. It is an occupational lung disease and a type ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermoset
In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening (" curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and may be promoted by high pressure, or mixing with a catalyst. Heat is not necessarily applied externally, but is often generated by the reaction of the resin with a curing agent (''catalyst'', ''hardener''). Curing results in chemical reactions that create extensive cross-linking between polymer chains to produce an infusible and insoluble polymer network. The starting material for making thermosets is usually malleable or liquid prior to curing, and is often designed to be molded into the final shape. It may also be used as an adhesive. Once hardened, a thermoset cannot be melted for reshaping, in contrast to thermoplastic polymers which are commonly produced and distributed in the form of pellets, and shaped into the final product form b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
M-Phenylenediamine
''m''-Phenylenediamine, also called 1,3-diaminobenzene, is an organic compound with the formula C6H4(NH2)2. It is an isomer of ''o''-phenylenediamine and ''p''-phenylenediamine. This aromatic diamine is a colourless solid that appears as needles, but turns red or purple on exposure to air due to formation of oxidation products. Samples often come as colourless flakes and may darken in storage. Production ''m''-Phenylenediamine is produced by hydrogenation of 1,3-dinitrobenzene. The dinitrobenzene is prepared by dinitration of benzene. Applications ''m''-Phenylenediamine is used in the preparation of various polymers including aramid fibers, epoxy resins, wire enamel coatings and polyurea elastomer An elastomer is a polymer with viscoelasticity (i.e. both viscosity and elasticity) and with weak intermolecular forces, generally low Young's modulus and high failure strain compared with other materials. The term, a portmanteau of ''elastic p ...s. Other uses for ''m''-phenyle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
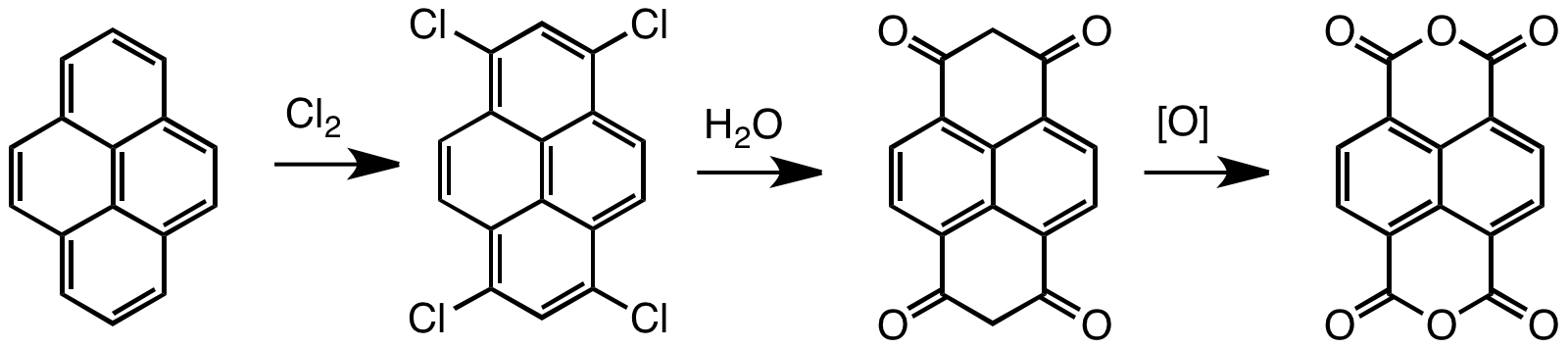
Naphthalene Tetracarboxylic Dianhydride
Naphthalenetetracarboxylic dianhydride (NTDA) is an organic compound related to naphthalene. The compound is a beige solid. NTDA is most commonly used as a precursor to naphthalenediimides (NDIs) (such as napthalenetetracarboxylic diimide), a family of compounds with many uses. Synthesis and structure Naphthalenetetracarboxylic dianhydride is prepared by oxidation of pyrene. Typical oxidants are chromic acid and chlorine. The unsaturated tetrachloride hydrolyzes to enols that tautomerize to the bis-dione, which in turn can be oxidized to the tetracarboxylic acid.F. Röhrscheid "Carboxylic Acids, Aromatic" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2012. Naphthalene diimides Symmetrical naphthalene diimides are synthesized by the condensation reaction of primary amines and the dianhydride. Unsymmetrical derivatives, i.e. those derived from two different amines, are obtained by hydrolysis of one of the two anhydride groups prior to the condensa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4,4'-Diaminodiphenyl Ether
4,4′-Oxydianiline (ODA) is an organic compound with the formula O( C6 H4 NH2)2. It is an ether derivative of aniline. This colourless solid is a useful monomer and cross-linking agent for polymers, especially the polyimides, such as Kapton. Uses 4,4′-Oxydianiline is used in the production of a wide variety of polymer resins. The primary use lies in the production of polyimide and poly(ester)imide resins. These resins are used for their temperature-resistant properties and are utilized in products including wire enamels, coatings, film, adhesives, insulating varnishes, coated fabrics, flame-retardant fibers, oil sealants and retainers, insulation for cables and printed circuits, and laminates and composite for aerospace vehicles. Other applications of 4,4′-oxydianiline include the production of poly(amide)imide resins (which are used in the manufacture of heat-resistant wire enamels and coatings), as an intermediate in the manufacture of epoxy resins and adhesives, and in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flexural Strength
Flexural strength, also known as modulus of rupture, or bend strength, or transverse rupture strength is a material property, defined as the stress in a material just before it yields in a flexure test. The transverse bending test is most frequently employed, in which a specimen having either a circular or rectangular cross-section is bent until fracture or yielding using a three-point flexural test technique. The flexural strength represents the highest stress experienced within the material at its moment of yield. It is measured in terms of stress, here given the symbol \sigma. Introduction When an object is formed of a single material, like a wooden beam or a steel rod, is bent (Fig. 1), it experiences a range of stresses across its depth (Fig. 2). At the edge of the object on the inside of the bend (concave face) the stress will be at its maximum compressive stress value. At the outside of the bend (convex face) the stress will be at its maximum tensile value. These in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Infrared Spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance (or transmittance) on the vertical axis vs. frequency, wavenumber or wavelength on the horizontal axis. Typical units of wavenumber used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers (formerly called "microns"), symbol μm, which are related to the wavenumber in a reciprocal way. A com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_V1.png)