|
Polyimide Formation (schematic) V1
Polyimide (sometimes abbreviated PI) is a polymer containing imide groups belonging to the class of high-performance plastics. With their high heat-resistance, polyimides enjoy diverse applications in roles demanding rugged organic materials, e.g. high temperature fuel cells, displays, and various military roles. A classic polyimide is Kapton, which is produced by condensation of pyromellitic dianhydride and 4,4'-Oxydianiline, 4,4'-oxydianiline.Wright, Walter W. and Hallden-Abberton, Michael (2002) "Polyimides" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim. History The first polyimide was discovered in 1908 by Bogart and Renshaw. They found that 4-amino phthalic anhydride does not melt when heated but does release water upon the formation of a high molecular weight polyimide. The first semialiphatic polyimide was prepared by Edward and Robinson by melt fusion of diamines and tetra acids or diamines and diacids / diester. However, the first polyimide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyimide
Polyimide (sometimes abbreviated PI) is a polymer containing imide groups belonging to the class of high-performance plastics. With their high heat-resistance, polyimides enjoy diverse applications in roles demanding rugged organic materials, e.g. high temperature fuel cells, displays, and various military roles. A classic polyimide is Kapton, which is produced by condensation of pyromellitic dianhydride and 4,4'-oxydianiline.Wright, Walter W. and Hallden-Abberton, Michael (2002) "Polyimides" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim. History The first polyimide was discovered in 1908 by Bogart and Renshaw. They found that 4-amino phthalic anhydride does not melt when heated but does release water upon the formation of a high molecular weight polyimide. The first semialiphatic polyimide was prepared by Edward and Robinson by melt fusion of diamines and tetra acids or diamines and diacids / diester. However, the first polyimide of significant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isocyanate
In organic chemistry, isocyanate is the functional group with the formula . Organic compounds that contain an isocyanate group are referred to as isocyanates. An organic compound with two isocyanate groups is known as a diisocyanate. Diisocyanates are manufactured for the production of polyurethanes, a class of polymers. Isocyanates should not be confused with cyanate esters and isocyanides, very different families of compounds. The cyanate (cyanate ester) functional group () is arranged differently from the isocyanate group (). Isocyanides have the connectivity , lacking the oxygen of the cyanate groups. Structure and bonding In terms of bonding, isocyanates are closely related to carbon dioxide (CO2) and carbodiimides (C(NR)2). The C−N=C=O unit that defines isocyanates is planar, and the N=C=O linkage is nearly linear. In phenyl isocyanate, the C=N and C=O distances are respectively 1.195 and 1.173 Å. The C-N=C angle is 134.9° and the N=C=O angle is 173.1°. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Creep (deformation)
In materials science, creep (sometimes called cold flow) is the tendency of a solid material to move slowly or deform permanently under the influence of persistent mechanical stresses. It can occur as a result of long-term exposure to high levels of stress that are still below the yield strength of the material. Creep is more severe in materials that are subjected to heat for long periods and generally increases as they near their melting point. The rate of deformation is a function of the material's properties, exposure time, exposure temperature and the applied structural load. Depending on the magnitude of the applied stress and its duration, the deformation may become so large that a component can no longer perform its function – for example creep of a turbine blade could cause the blade to contact the casing, resulting in the failure of the blade. Creep is usually of concern to engineers and metallurgists when evaluating components that operate under high stresses or hig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermoset Polymer Matrix
A thermoset polymer matrix is a synthetic polymer reinforcement where polymers act as binder or matrix to secure in place incorporated particulates, fibres or other reinforcements. They were first developed for structural applications, such as glass-reinforced plastic radar domes on aircraft and graphite-epoxy payload bay doors on the Space Shuttle. They were first used after World War II, and continuing research has led to an increased range of thermoset resins, polymers or plastics, as well as engineering grade thermoplastics. They were all developed for use in the manufacture of polymer composites with enhanced and longer-term service capabilities. Thermoset polymer matrix technologies also find use in a wide diversity of non-structural industrial applications. The foremost types of thermosetting polymers used in structural composites are benzoxazine resins, bis-maleimide resins (BMI), cyanate ester resins, epoxy (epoxide) resins, phenolic (PF) resins, unsaturated polyester ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
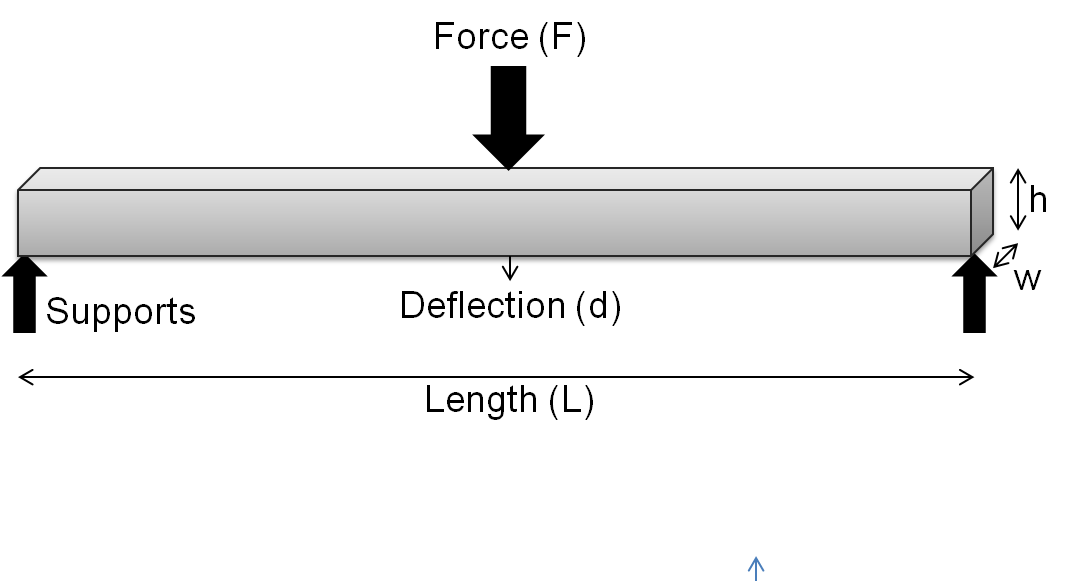
Flexural Modulus
In mechanics, the flexural modulus or bending modulus is an intensive property that is computed as the ratio of stress to strain in flexural deformation, or the tendency for a material to resist bending. It is determined from the slope of a stress-strain curve produced by a flexural test (such as the ASTM D790), and uses units of force per area. The flexural modulus defined using the 2-point (cantilever) and 3-point bend tests assumes a linear stress strain response. For a 3-point test of a rectangular beam behaving as an isotropic linear material, where ''w'' and ''h'' are the width and height of the beam, ''I'' is the second moment of area of the beam's cross-section, ''L'' is the distance between the two outer supports, and ''d'' is the deflection due to the load ''F'' applied at the middle of the beam, the flexural modulus: : E_ = \frac From elastic beam theory :d = \frac and for rectangular beam : I = \fracwh^3 thus E_ = E (Elastic modulus) For very small strains ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flexural Strength
Flexural strength, also known as modulus of rupture, or bend strength, or transverse rupture strength is a material property, defined as the stress in a material just before it yields in a flexure test. The transverse bending test is most frequently employed, in which a specimen having either a circular or rectangular cross-section is bent until fracture or yielding using a three-point flexural test technique. The flexural strength represents the highest stress experienced within the material at its moment of yield. It is measured in terms of stress, here given the symbol \sigma. Introduction When an object is formed of a single material, like a wooden beam or a steel rod, is bent (Fig. 1), it experiences a range of stresses across its depth (Fig. 2). At the edge of the object on the inside of the bend (concave face) the stress will be at its maximum compressive stress value. At the outside of the bend (convex face) the stress will be at its maximum tensile value. These in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass Fiber
Glass fiber ( or glass fibre) is a material consisting of numerous extremely fine fibers of glass. Glassmakers throughout history have experimented with glass fibers, but mass manufacture of glass fiber was only made possible with the invention of finer machine tooling. In 1893, Edward Drummond Libbey exhibited a dress at the World's Columbian Exposition incorporating glass fibers with the diameter and texture of silk fibers. Glass fibers can also occur naturally, as Pele's hair. Glass wool, which is one product called "fiberglass" today, was invented some time between 1932 to 1933 by Games Slayter of Owens-Illinois, as a material to be used as thermal building insulation. It is marketed under the trade name Fiberglas, which has become a genericized trademark. Glass fiber when used as a thermal insulating material is specially manufactured with a bonding agent to trap many small air cells, resulting in the characteristically air-filled low-density "glass wool" family of pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon (fiber)
Carbon fibers or carbon fibres (alternatively CF, graphite fiber or graphite fibre) are fibers about in diameter and composed mostly of carbon atoms. Carbon fibers have several advantages: high stiffness, high tensile strength, high strength to weight ratio, high chemical resistance, high-temperature tolerance, and low thermal expansion. These properties have made carbon fiber very popular in aerospace, civil engineering, military, motorsports, and other competition sports. However, they are relatively expensive compared to similar fibers, such as glass fiber, basalt fibers, or plastic fibers. To produce a carbon fiber, the carbon atoms are bonded together in crystals that are more or less aligned parallel to the fiber's long axis as the crystal alignment gives the fiber a high strength-to-volume ratio (in other words, it is strong for its size). Several thousand carbon fibers are bundled together to form a tow, which may be used by itself or woven into a fabric. Carbon f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Infrared Spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance (or transmittance) on the vertical axis vs. frequency, wavenumber or wavelength on the horizontal axis. Typical units of wavenumber used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers (formerly called "microns"), symbol μm, which are related to the wavenumber in a reciprocal way. A com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
M-Phenylenediamine
''m''-Phenylenediamine, also called 1,3-diaminobenzene, is an organic compound with the formula C6H4(NH2)2. It is an isomer of ''o''-phenylenediamine and ''p''-phenylenediamine. This aromatic diamine is a colourless solid that appears as needles, but turns red or purple on exposure to air due to formation of oxidation products. Samples often come as colourless flakes and may darken in storage. Production ''m''-Phenylenediamine is produced by hydrogenation of 1,3-dinitrobenzene. The dinitrobenzene is prepared by dinitration of benzene. Applications ''m''-Phenylenediamine is used in the preparation of various polymers including aramid fibers, epoxy resins, wire enamel coatings and polyurea elastomer An elastomer is a polymer with viscoelasticity (i.e. both viscosity and elasticity) and with weak intermolecular forces, generally low Young's modulus and high failure strain compared with other materials. The term, a portmanteau of ''elastic p ...s. Other uses for ''m''-phenyle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4,4'-Diaminodiphenyl Ether
4,4′-Oxydianiline (ODA) is an organic compound with the formula O( C6 H4 NH2)2. It is an ether derivative of aniline. This colourless solid is a useful monomer and cross-linking agent for polymers, especially the polyimides, such as Kapton. Uses 4,4′-Oxydianiline is used in the production of a wide variety of polymer resins. The primary use lies in the production of polyimide and poly(ester)imide resins. These resins are used for their temperature-resistant properties and are utilized in products including wire enamels, coatings, film, adhesives, insulating varnishes, coated fabrics, flame-retardant fibers, oil sealants and retainers, insulation for cables and printed circuits, and laminates and composite for aerospace vehicles. Other applications of 4,4′-oxydianiline include the production of poly(amide)imide resins (which are used in the manufacture of heat-resistant wire enamels and coatings), as an intermediate in the manufacture of epoxy resins and adhesives, and in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
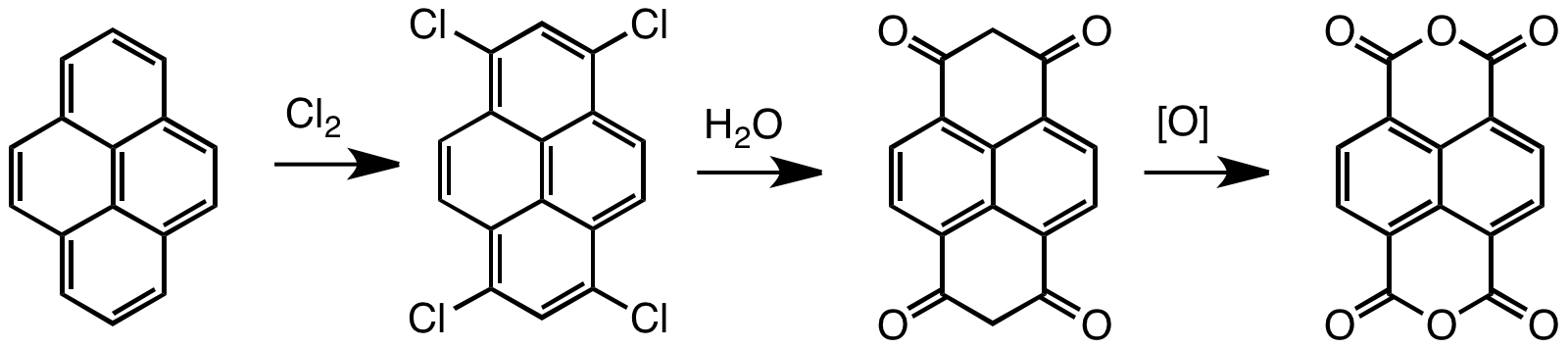
Naphthalene Tetracarboxylic Dianhydride
Naphthalenetetracarboxylic dianhydride (NTDA) is an organic compound related to naphthalene. The compound is a beige solid. NTDA is most commonly used as a precursor to naphthalenediimides (NDIs) (such as napthalenetetracarboxylic diimide), a family of compounds with many uses. Synthesis and structure Naphthalenetetracarboxylic dianhydride is prepared by oxidation of pyrene. Typical oxidants are chromic acid and chlorine. The unsaturated tetrachloride hydrolyzes to enols that tautomerize to the bis-dione, which in turn can be oxidized to the tetracarboxylic acid.F. Röhrscheid "Carboxylic Acids, Aromatic" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2012. Naphthalene diimides Symmetrical naphthalene diimides are synthesized by the condensation reaction of primary amines and the dianhydride. Unsymmetrical derivatives, i.e. those derived from two different amines, are obtained by hydrolysis of one of the two anhydride groups prior to the condensa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_V1.png)