|
Phosphorus Halide
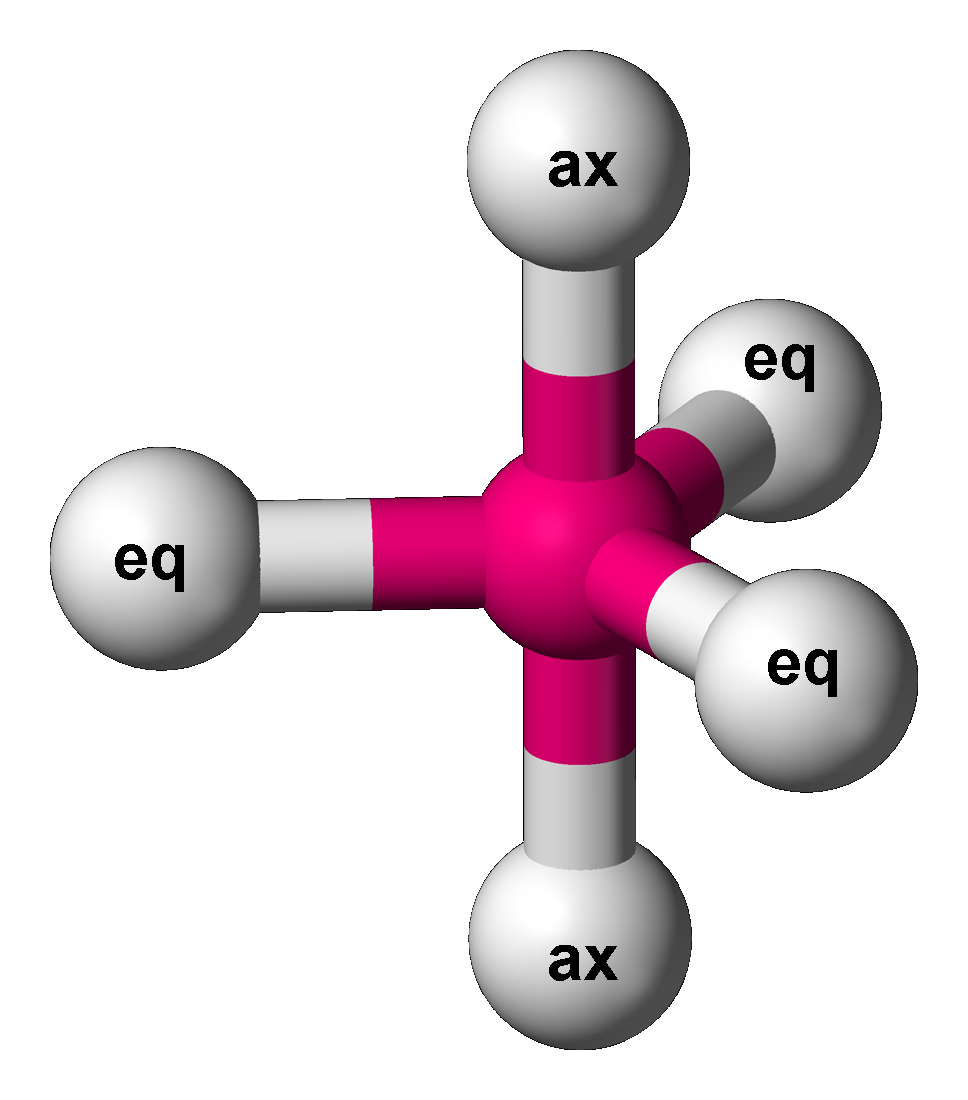
In chemistry, there are three series of binary phosphorus halides, containing phosphorus in the oxidation states +5, +3 and +2. All compounds have been described, in varying degrees of detail, although serious doubts have been cast on the existence of PI5.I. Tornieporth-Getting & T. Klapötke, ''J. Chem. Soc.'', ''Chem. Commun.'' 1990, 132. Mixed chalcogen halides also exist. Oxidation state +5 (PX5) In the gas phase the phosphorus pentahalides have trigonal bipyramidal molecular geometry as explained by VSEPR theory. Phosphorus pentafluoride is a relatively inert gas, notable as a mild Lewis acid and a fluoride ion acceptor. It is a fluxional molecule in which the axial (ax) and equatorial (eq) fluorine atoms interchange positions by the Berry pseudorotation mechanism. Phosphorus pentachloride, phosphorus pentabromide, and phosphorus heptabromide are ionic in the solid and liquid states; PCl5 is formulated as PCl4+PCl6–, but in contrast, PBr5 is formulated as PBr4+ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a Chemical reaction, reaction with other Chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
VSEPR Theory
Valence shell electron pair repulsion (VSEPR) theory ( , ), is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Nyholm. The premise of VSEPR is that the valence electron pairs surrounding an atom tend to repel each other and will, therefore, adopt an arrangement that minimizes this repulsion. This in turn decreases the molecule's energy and increases its stability, which determines the molecular geometry. Gillespie has emphasized that the electron-electron repulsion due to the Pauli exclusion principle is more important in determining molecular geometry than the electrostatic repulsion. The insights of VSEPR theory are derived from topological analysis of the electron density of molecules. Such quantum chemical topology (QCT) methods include the electron localization function (ELF) and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Tricyanide
Phosphorus tricyanide is an inorganic compound with the chemical formula P(CN)3. It can be produced by the reaction of phosphorus trichloride and trimethyl(iso)cyanosilane. The reaction of phosphorus tribromide and silver cyanide in diethyl ether Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable li ... produce phosphorus tricyanide too. Its thermal decomposition can produce graphite phase C3N3P. Phosphorus tricyanide reacts with Re(CO)5FBF3 to form (BF4)3. References {{Cyanides Phosphorus(III) compounds Cyanides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Triiodide
Phosphorus triiodide (PI3) is an inorganic compound with the formula PI3. A red solid, it is a common misconception that PI3 is too unstable to be stored; it is, in fact, commercially available. It is widely used in organic chemistry for converting alcohols to alkyl iodides. It is also a powerful reducing agent. Note that phosphorus also forms a lower iodide, P2I4, but the existence of PI5 is doubtful at room temperature. Properties PI3 has a low dipole moment in carbon disulfide solution, because the P-I bond has almost no dipole. The P-I bond is also weak; PI3 is much less stable than PBr3 and PCl3, with a standard enthalpy of formation for PI3 of only −46 kJ/ mol (solid). The phosphorus atom has an NMR chemical shift of 178 ppm (downfield of H3PO4). Reactions Phosphorus triiodide reacts vigorously with water, producing phosphorous acid (H3PO3) and hydroiodic acid (HI), along with smaller amounts of phosphine and various P-P-containing compounds. Alcohols likewise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Tribromide
Phosphorus tribromide is a colourless liquid with the formula P Br3. The liquid fumes in moist air due to hydrolysis and has a penetrating odour. It is used in the laboratory for the conversion of alcohols to alkyl bromides. Preparation PBr3 is prepared by treating red phosphorus with bromine. An excess of phosphorus is used in order to prevent formation of PBr5: :2 P + 3 Br2 → 2 PBr3 Because the reaction is highly exothermic, it is often conducted in the presence of a diluent such as PBr3. Reactions Phosphorus tribromide, like PCl3 and PF3, has both properties of a Lewis base and a Lewis acid. For example, with a Lewis acid such as boron tribromide it forms stable 1 :1 adducts such as Br3B · PBr3. At the same time PBr3 can react as an electrophile or Lewis acid in many of its reactions, for example with amines. The most important reaction of PBr3 is with alcohols, where it replaces an OH group with a bromine atom to produce an alkyl bromide. All three bromides ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Trichloride
Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts readily with water to release hydrogen chloride. History Phosphorus trichloride was first prepared in 1808 by the French chemists Joseph Louis Gay-Lussac and Louis Jacques Thénard by heating calomel (Hg2Cl2) with phosphorus. Later during the same year, the English chemist Humphry Davy produced phosphorus trichloride by burning phosphorus in chlorine gas. Preparation World production exceeds one-third of a million tonnes. Phosphorus trichloride is prepared industrially by the reaction of chlorine with white phosphorus, using phosphorus trichloride as the solvent. In this continuous process PCl3 is removed as it is formed in order to avoid the formation of PCl5. :P4 + 6 Cl2 → 4 PCl3 Structure and spectroscopy It has a trig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Trifluoride
Phosphorus trifluoride (formula P F3), is a colorless and odorless gas. It is highly toxic and reacts slowly with water. Its main use is as a ligand in metal complexes. As a ligand, it parallels carbon monoxide in metal carbonyls, and indeed its toxicity is due to its binding with the iron in blood hemoglobin in a similar way to carbon monoxide. Physical properties Phosphorus trifluoride has an F−P−F bond angle of approximately 96.3°. Gaseous PF3 has a standard enthalpy of formation of −945 kJ/mol (−226 kcal/ mol). The phosphorus atom has a nuclear magnetic resonance chemical shift of 97 ppm (downfield of H3PO4). Properties Phosphorus trifluoride hydrolyzes especially at high pH, but it is less hydrolytically sensitive than phosphorus trichloride. It does not attack glass except at high temperatures, and anhydrous potassium hydroxide may be used to dry it with little loss. With hot metals, phosphides and fluorides are formed. With Lewis bases such a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Dipole Moment
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end. Polar molecules must contain one or more polar bonds due to a difference in electronegativity between the bonded atoms. Molecules containing polar bonds have no molecular polarity if the bond dipoles cancel each other out by symmetry. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points. Polarity of bonds Not all atoms attract electrons with the same force. The amount of "pull" an atom exerts on its electrons is called its electronegativity. Atoms with high electronegativitiessuch as fluorine, oxygen, and nitrogenexert a greater pull on electrons than atoms with lower electronegativities such as alkali metals and alkaline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tribromide
Tribromide is the anion with the chemical formula Br3−, or salts containing it: * Tetrabutylammonium tribromide * Tetrabromophosphonium tribromide * Pyridinium perbromide Sodium and potassium tribromides can be prepared by reacting NaBr or KBr with aqueous bromine. : Br− + Br2 → Br3− Tribromide may also refer to binary chemical compounds containing three bromine atoms: * Aluminium tribromide, AlBr3 * Antimony tribromide, SbBr3 * Arsenic tribromide, AsBr3 * Bismuth tribromide, BiBr3 * Boron tribromide, BBr3 * Chromium tribromide, CrBr3 * Erbium tribromide, ErBr3 * Europium tribromide, EuBr3 * Ferric tribromide, FeBr3 * Gallium tribromide, GaBr3 * Gold tribromide, AuBr3 or Au2Br6 * Indium tribromide, InBr3 * Molybdenum tribromide, MoBr3 * Nitrogen tribromide, NBr3 * Phosphorus tribromide Phosphorus tribromide is a colourless liquid with the formula P Br3. The liquid fumes in moist air due to hydrolysis and has a penetrating odour. It is used in the labora ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Heptabromide
Phosphorus heptabromide is an inorganic compound with the formula PBr7. It is one of the phosphorus bromides. At normal conditions, it forms red prismatic crystals. PBr7 can be prepared by the sublimation of a mixture of phosphorus pentabromide and bromine. :PBr5 + Br2 → PBr7 The structure consists of a PBr4+ cation paired with a tribromide (Br3–) anion, and the tribromide is non-symmetric. See also * Phosphorus tribromide Phosphorus tribromide is a colourless liquid with the formula P Br3. The liquid fumes in moist air due to hydrolysis and has a penetrating odour. It is used in the laboratory for the conversion of alcohols to alkyl bromides. Preparation PBr3 ... * Phosphorus pentabromide References Phosphorus bromides Polyhalides Phosphonium compounds {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Pentabromide
Phosphorus pentabromide is a reactive, yellow solid of formula P Br5, which has the structure PBr4+ Br− in the solid state but in the vapor phase is completely dissociated to PBr3 and Br2. Rapid cooling of this phase to 15 K leads to formation of the ionic species phosphorus heptabromide ( Br4sup>+ r3sup>−). It can be used in organic chemistry to convert carboxylic acids to acyl bromides. It is highly corrosive. It decomposes above 100 °C to give phosphorus tribromide and bromine Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table ( halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simi ...: :PBr5 → PBr3 + Br2 Reversing this equilibrium to generate PBr5 by addition of Br2 to PBr3 is difficult in practice because the product is susceptible to further addition to yield phosphorus heptabromide (PBr7). References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berry Pseudorotation Mechanism
The Berry mechanism, or Berry pseudorotation mechanism, is a type of vibration causing molecules of certain geometries to isomerize by exchanging the two axial ligands (see Figure at right) for two of the equatorial ones. It is the most widely accepted mechanism for pseudorotation and most commonly occurs in trigonal bipyramidal molecules such as PF5, though it can also occur in molecules with a square pyramidal geometry. The Berry mechanism is named after R. Stephen Berry, who first described this mechanism in 1960.RS Berry, 1960, "Correlation of rates of intramolecular tunneling processes, with application to some Group V compounds," ''J. Chem. Phys.'' 32:933-938, DOI 10.1063/1.1730820; seo accessed 28 May 2014M Cass, KK Hii & HS Rzepa, 2005, "Mechanisms that interchange axial and equatorial atoms in fluxional processes: Illustration of the Berry pseudorotation, the turnstile and the lever mechanisms via animation of transition state normal vibrational modes", ''J. Chem. Educ. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


platinum(0)-from-xtal-2008-3D-balls.png)