|
Peptide Amphiphile
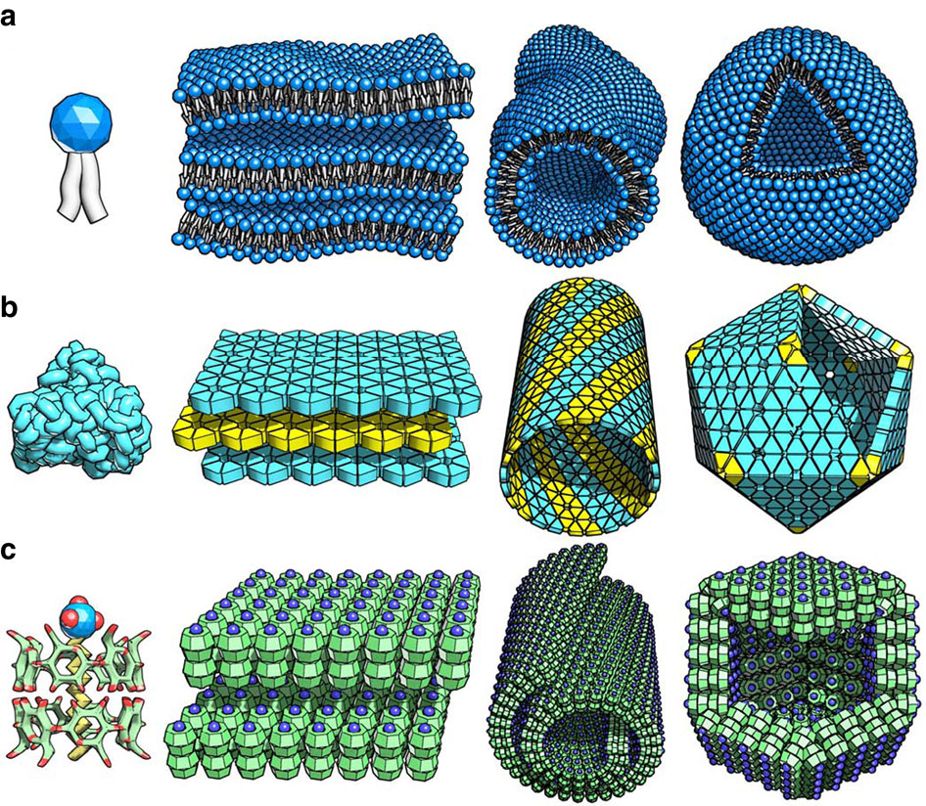
Peptide amphiphiles (PAs) are peptide-based molecules that self-assemble into supramolecular nanostructures including; spherical micelles, twisted ribbons, and high-aspect-ratio nanofibers. A peptide amphiphile typically comprises a hydrophilic peptide sequence attached to a lipid tail, i.e. a hydrophobic alkyl chain with 10 to 16 carbons. Therefore, they can be considered a type of lipopeptide. A special type of PA, is constituted by alternating charged and neutral residues, in a repeated pattern, such as RADA16-I. The PAs were developed in the 1990s and the early 2000s and could be used in various medical areas including: nanocarriers, nanodrugs, and imaging agents. However, perhaps their main potential is in regenerative medicine to culture and deliver cells and growth factors. History Peptide amphiphiles were developed in the 1990s. They were first described by the group of Matthew Tirrell in 1995. These first reported PA molecules were composed of two domains: one of lipoph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide Amphiphiles Possible Structures Simplified Scheme
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic pe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vesicle (biology And Chemistry)
In cell biology, a vesicle is a structure within or outside a cell, consisting of liquid or cytoplasm enclosed by a lipid bilayer. Vesicles form naturally during the processes of secretion (exocytosis), uptake ( endocytosis) and transport of materials within the plasma membrane. Alternatively, they may be prepared artificially, in which case they are called liposomes (not to be confused with lysosomes). If there is only one phospholipid bilayer, the vesicles are called ''unilamellar liposomes''; otherwise they are called ''multilamellar liposomes''. The membrane enclosing the vesicle is also a lamellar phase, similar to that of the plasma membrane, and intracellular vesicles can fuse with the plasma membrane to release their contents outside the cell. Vesicles can also fuse with other organelles within the cell. A vesicle released from the cell is known as an extracellular vesicle. Vesicles perform a variety of functions. Because it is separated from the cytosol, the inside of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptides
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic peptides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogel
A hydrogel is a crosslinked hydrophilic polymer that does not dissolve in water. They are highly absorbent yet maintain well defined structures. These properties underpin several applications, especially in the biomedical area. Many hydrogels are synthetic, but some are derived from nature. The term 'hydrogel' was coined in 1894. Chemistry Classification The crosslinks which bond the polymers of a hydrogel fall under two general categories: physical and chemical. Chemical hydrogels have covalent cross-linking bonds, whereas physical hydrogels have non-covalent bonds. Chemical hydrogels result in strong irreversible gels due to the covalent bonding, and they may also possess harmful properties which makes them unfavourable for medical applications. Physical hydrogels on the other hand have high biocompatibility, aren’t toxic, and are also easily reversible, by simply changing an external stimulus such as pH or temperature; thus they are favourable for use in medical applicat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomimetic Material
Biomimetic materials are materials developed using inspiration from nature. This may be useful in the design of composite materials. Natural structures have inspired and innovated human creations. Notable examples of these natural structures include: honeycomb structure of the beehive, strength of spider silks, bird flight mechanics, and shark skin water repellency. The etymological roots of the neologism "biomimetic" derive from Greek, since means "life" and means "imitative". Tissue engineering Biomimetic materials in tissue engineering are materials that have been designed such that they elicit specified cellular responses mediated by interactions with scaffold-tethered peptides from extracellular matrix (ECM) proteins; essentially, the incorporation of cell-binding peptides into biomaterials via chemical or physical modification.Shin, H., S. Jo, and A.G. Mikos, ''Biomimetic materials for tissue engineering''. Biomaterials, 2003. 24: p. 4353-5364. Amino acids located within the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biopolymer
Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, classified according to the monomers used and the structure of the biopolymer formed: polynucleotides, polypeptides, and polysaccharides. The Polynucleotides, RNA and DNA, are long polymers of nucleotides. Polypeptides include proteins and shorter polymers of amino acids; some major examples include collagen, actin, and fibrin. Polysaccharides are linear or branched chains of sugar carbohydrates; examples include starch, cellulose and alginate. Other examples of biopolymers include natural rubbers (polymers of isoprene), suberin and lignin (complex polyphenolic polymers), cutin and cutan (complex polymers of long-chain fatty acids) and melanin. In addition to their many essential roles in living organisms, biopolymers have applications in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heparin Sulfate
Heparan sulfate (HS) is a linear polysaccharide found in all animal tissues. It occurs as a proteoglycan (HSPG, i.e. Heparan Sulfate ProteoGlycan) in which two or three HS chains are attached in close proximity to cell surface or extracellular matrix proteins. It is in this form that HS binds to a variety of protein ligands, including Wnt, and regulates a wide range of biological activities, including developmental processes, angiogenesis, blood coagulation, abolishing detachment activity by GrB (Granzyme B), and tumour metastasis. HS has also been shown to serve as cellular receptor for a number of viruses, including the respiratory syncytial virus. One study suggests that cellular heparan sulfate has a role in SARS-CoV-2 Infection, particularly when the virus attaches with ACE2. Proteoglycans The major cell membrane HSPGs are the transmembrane syndecans and the glycosylphosphatidylinositol (GPI) anchored glypicans. Other minor forms of membrane HSPG include betaglycan and the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
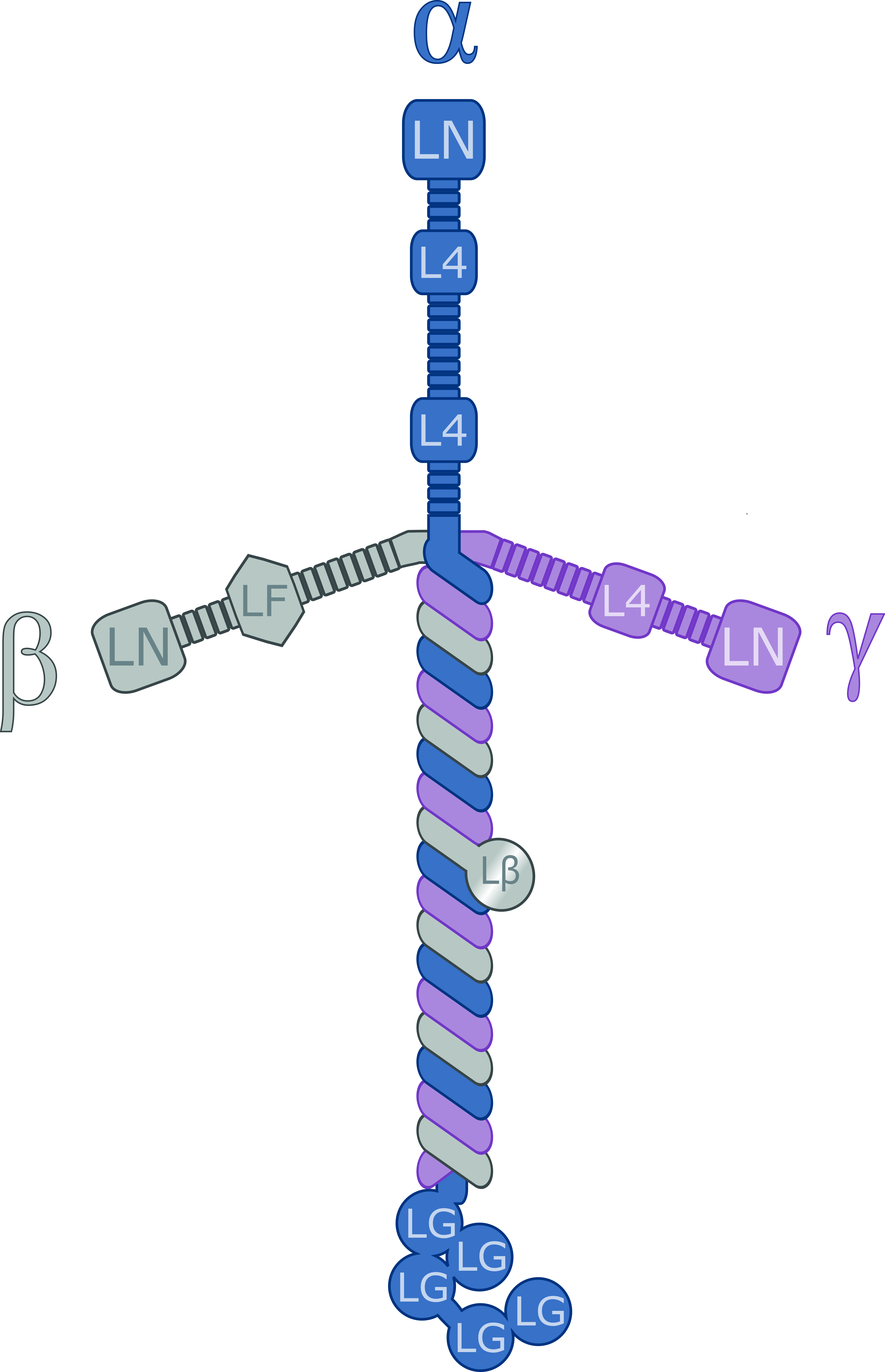
Laminin
Laminins are a family of glycoproteins of the extracellular matrix of all animals. They are major components of the basal lamina (one of the layers of the basement membrane), the protein network foundation for most cells and organs. The laminins are an important and biologically active part of the basal lamina, influencing cell differentiation, migration, and adhesion. Laminins are heterotrimeric proteins with a high molecular mass (~400 to ~900 kDa). They contain three different chains (α, β and γ) encoded by five, four, and three paralogous genes in humans, respectively. The laminin molecules are named according to their chain composition. Thus, laminin-511 contains α5, β1, and γ1 chains. Fourteen other chain combinations have been identified ''in vivo''. The trimeric proteins intersect to form a cross-like structure that can bind to other cell membrane and extracellular matrix molecules. The three shorter arms are particularly good at binding to other laminin molecules, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
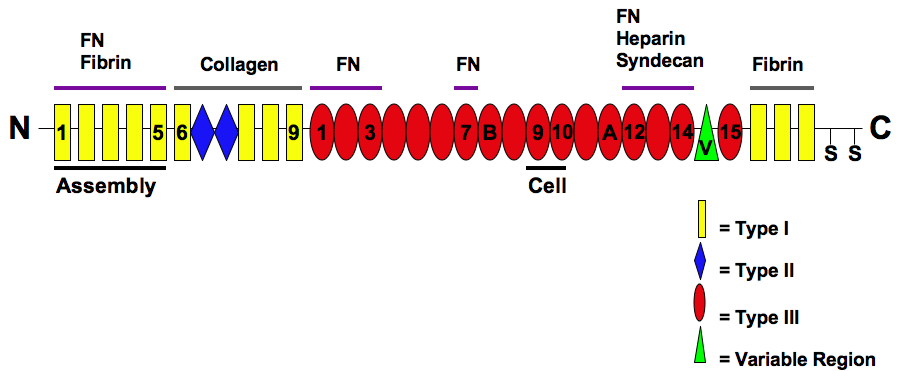
Fibronectin
Fibronectin is a high- molecular weight (~500-~600 kDa) glycoprotein of the extracellular matrix that binds to membrane-spanning receptor proteins called integrins. Fibronectin also binds to other extracellular matrix proteins such as collagen, fibrin, and heparan sulfate proteoglycans (e.g. syndecans). Fibronectin exists as a protein dimer, consisting of two nearly identical monomers linked by a pair of disulfide bonds. The fibronectin protein is produced from a single gene, but alternative splicing of its pre-mRNA leads to the creation of several isoforms. Two types of fibronectin are present in vertebrates: * soluble plasma fibronectin (formerly called "cold-insoluble globulin", or CIg) is a major protein component of blood plasma (300 μg/ml) and is produced in the liver by hepatocytes. * insoluble cellular fibronectin is a major component of the extracellular matrix. It is secreted by various cells, primarily fibroblasts, as a soluble protein dimer and is then ass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RGD Motif
Arginylglycylaspartic acid (RGD) is the most common peptide motif responsible for cell adhesion to the extracellular matrix (ECM), found in species ranging from ''Drosophila'' to humans. Cell adhesion proteins called integrins recognize and bind to this sequence, which is found within many matrix proteins, including fibronectin, fibrinogen, vitronectin, osteopontin, and several other adhesive extracellular matrix proteins. The discovery of RGD and elucidation of how RGD binds to integrins has led to the development of a number of drugs and diagnostics, while the peptide itself is used ubiquitously in bioengineering. Depending on the application and the integrin targeted, RGD can be chemically modified or replaced by a similar peptide which promotes cell adhesion. Discovery RGD was identified as the minimal recognition sequence within fibronectin required for cell attachment by Ruoslahti and Pierschbacher in the early 1980s. To do this, the authors synthesized various peptides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Self-assembly
Self-assembly is a process in which a disordered system of pre-existing components forms an organized structure or pattern as a consequence of specific, local interactions among the components themselves, without external direction. When the constitutive components are molecules, the process is termed molecular self-assembly. Self-assembly can be classified as either static or dynamic. In ''static'' self-assembly, the ordered state forms as a system approaches equilibrium, reducing its free energy. However, in ''dynamic'' self-assembly, patterns of pre-existing components organized by specific local interactions are not commonly described as "self-assembled" by scientists in the associated disciplines. These structures are better described as "self-organized", although these terms are often used interchangeably. Self-assembly in chemistry and materials science Self-assembly in the classic sense can be defined as ''the spontaneous and reversible organization of molec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (the extracellular space). The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols (a lipid component) interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of cells and organelles, being selectively permeable to ions a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |