|
Pentamethylcyclopentadienyl Iridium Dichloride Dimer
Pentamethylcyclopentadienyl iridium dichloride dimer is an organometallic compound with the formula C5(CH3)5IrCl2)sub>2, commonly abbreviated p*IrCl2sub>2 This bright orange air-stable diamagnetic solid is a reagent in organometallic chemistry. Structure The compound has C2h symmetry. Each metal is pseudo-octahedral. The terminal and bridging Ir-Cl bonds have the lengths 2.39 and 2.45 Å, respectively. Preparation, reactions Pentamethylcyclopentadienyl iridium dichloride dimer was first prepared by the reaction of hydrated iridium trichloride with hexamethyl Dewar benzene. More conveniently, the compound is prepared by the reaction of hydrated iridium trichloride and pentamethylcyclopentadiene in hot methanol, from which the product precipitates :2 Cp*H + 2 IrCl3(H2O)3 → p*IrCl2sub>2 + 2 HCl + 6 H2O The Ir-'' μ''-Cl bonds are labile and can be cleaved to give a variety of adducts of the general formula Cp*IrCl2L. Such adducts undergo further substitution to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organometallic Chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide (metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term " metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are repres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azide
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant application of azides is as a propellant in air bags. Preparation Sodium azide is made industrially by the reaction of nitrous oxide, with sodium amide in liquid ammonia as solvent: : Many inorganic azides can be prepared directly or indirectly from sodium azide. For example, lead azide, used in detonators, may be prepared from the metathesis reaction between lead nitrate and sodium azide. An alternative route is direct reaction of the metal with silver azide dissolved in liquid ammonia. Some azides are produced by treating the carbonate salts with hydrazoic acid. Bonding Azide is isoelectronic with carbon dioxide , cyanate , nitrous oxide , nitronium ion and cyanogen fluoride NCF. Per valence bond theory, azide can be described ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimers (chemistry)
Dimer may refer to: * Dimer (chemistry), a chemical structure formed from two similar sub-units ** Protein dimer, a protein quaternary structure ** d-dimer * Dimer model, an item in statistical mechanics, based on ''domino tiling'' * Julius Dimer Julius Dimer (1 August 1871 – 20 October 1945) was a German chess master. At the beginning of his career, he played in several mini tournaments (''Quadrangular'') in Germany; at Altona 1897, Elmshorn 1898, Munich 1900, Kiel 1901, Hamburg 1903, ... (1871–1945), German chess master See also * Dimery (botany), having two parts in a distinct whorl of a plant structure * Di (other), a prefix * Dymer (other) * -mer, a suffix * Oligomer * Peierls transition, sometimes called dimerization {{Disambiguation, surname ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metallocenes
A metallocene is a compound typically consisting of two cyclopentadienyl anions (, abbreviated Cp) bound to a metal center (M) in the oxidation state II, with the resulting general formula Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride, vanadocene dichloride. Certain metallocenes and their derivatives exhibit catalytic properties, although metallocenes are rarely used industrially. Cationic group 4 metallocene derivatives related to p2ZrCH3sup>+ catalyze olefin polymerization. Some metallocenes consist of metal plus two cyclooctatetraenide anions (, abbreviated cot2−), namely the lanthanocenes and the actinocenes (uranocene and others). Metallocenes are a subset of a broader class of compounds called sandwich compounds. In the structure shown at right, the two pentagons are the cyclopentadienyl anions with circles inside them indicating they are aromatically stabilized. Here they are shown in a staggered conformation. Hist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentamethylcyclopentadienyl Rhodium Dichloride Dimer
Pentamethylcyclopentadienyl rhodium dichloride dimer is an organometallic compound with the formula C5(CH3)5RhCl2)sub>2, commonly abbreviated p*RhCl2sub>2 This dark red air-stable diamagnetic solid is a reagent in organometallic chemistry. Structure and preparation The compound has idealized C2h symmetry. Each metal centre is pseudo-octahedral. The compound is prepared by the reaction of rhodium trichloride trihydrate and pentamethylcyclopentadiene in hot methanol, from which the product precipitates: :2 Cp*H + 2 RhCl3(H2O)3 -> ce \text/chem> p*RhCl2sub>2 + 2 HCl + 6 H2O It was first prepared by the reaction of hydrated rhodium trichloride with hexamethyl Dewar benzene Reactions Reductive carbonylation gives p*Rh(CO)2 The Rh-'' μ''-Cl bonds are labile and cleave en route to a variety of adducts of the general formula Cp*RhCl2L. Treatment with silver ions in polar coordinating solvents cause ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bulky Cyclopentadienyl Ligands
In the area of organometallic chemistry, a bulky cyclopentadienyl ligand is jargon for a ligand of the type where R is a branched alkyl and ''n'' = 3 or 4. Representative examples are the tetraisopropyl derivative and the tris( ''tert''-butyl) derivative . These ligands are so large that their complexes behave differently from the pentamethylcyclopentadienyl analogues. Because they cannot closely approach the metal, these bulky ligands stabilize high spin complexes, such as (C5H2''t''Bu3)2Fe2I2. These large ligands stabilize highly unsaturated derivatives such as (C5H2''t''Bu3)2Fe2N2. Synthesis and reactions The (''tert''-butyl)cyclopentadiene is prepared by alkylation of cyclopentadiene with ''tert''-butyl bromide in the presence of sodium hydride and dibenzo-18-crown-6. The intermediate in this synthesis is di-''tert''-butylcyclopentadiene. This compound is conveniently prepared by alkylation of cyclobutadiene with tert-butyl bromide under phase-transfer condi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transfer Hydrogenation
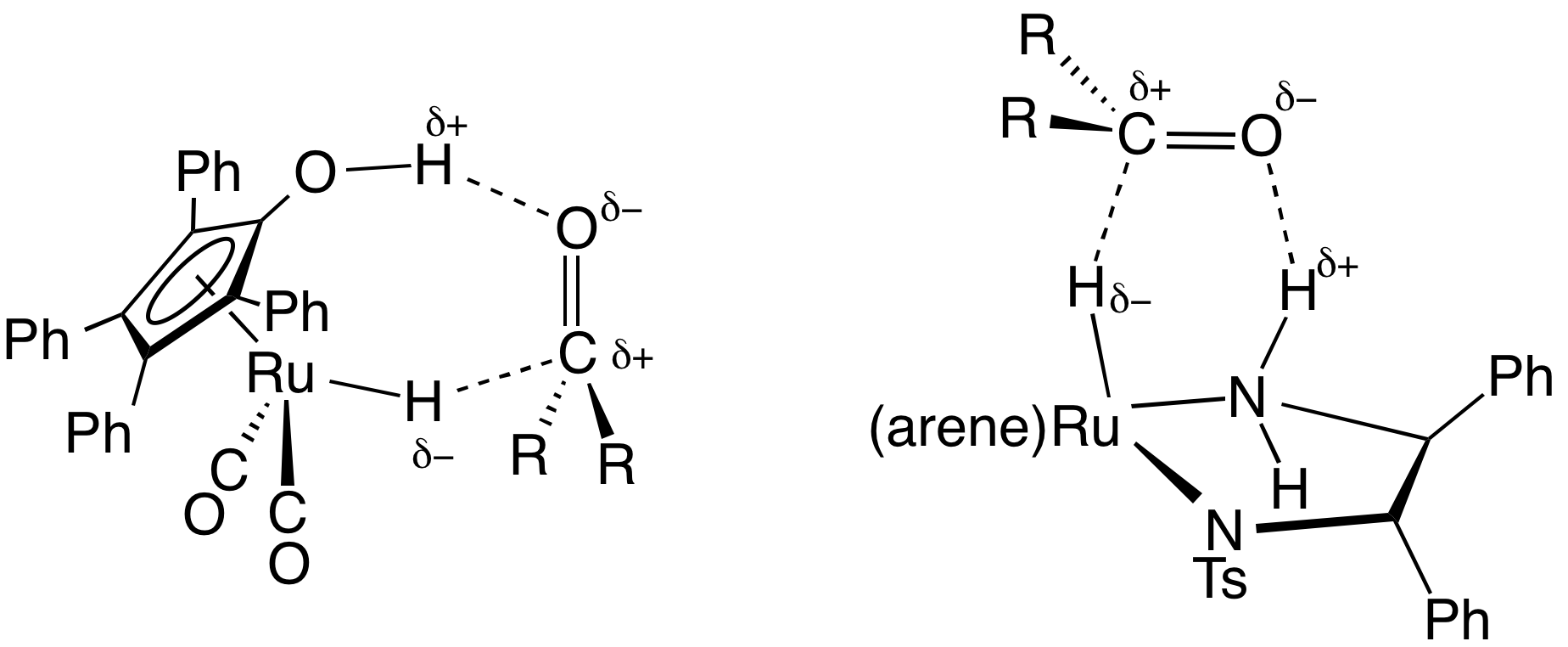
In chemistry, transfer hydrogenation is a chemical reaction involving the addition of hydrogen to a compound from a source other than molecular . It is applied in laboratory and industrial organic synthesis to saturate organic compounds and reduce ketones to alcohols, and imines to amines. It avoids the need for high-pressure molecular used in conventional hydrogenation. Transfer hydrogenation usually occurs at mild temperature and pressure conditions using organic or organometallic catalysts, many of which are chiral, allowing efficient asymmetric synthesis. It uses hydrogen donor compounds such as formic acid, isopropanol or dihydroanthracene, dehydrogenating them to , acetone, or anthracene respectively. Often, the donor molecules also function as solvents for the reaction. A large scale application of transfer hydrogenation is coal liquefaction using "donor solvents" such as tetralin. Organometallic catalysts In the area of organic synthesis, a useful family of hydrogen-trans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borohydride
Borohydride refers to the anion , which is also called tetrahydroborate, and its salts. Borohydride or hydroborate is also the term used for compounds containing , where ''n'' is an integer from 0 to 3, for example cyanoborohydride or cyanotrihydroborate and triethylborohydride or triethylhydroborate . Borohydrides find wide use as reducing agents in organic synthesis. The most important borohydrides are lithium borohydride and sodium borohydride, but other salts are well known (see Table). Tetrahydroborates are also of academic and industrial interest in inorganic chemistry. History Alkali metal borohydrides were first described in 1940 by Hermann Irving Schlesinger and Herbert C. Brown. They synthesized lithium borohydride from diborane : :, where M = Li, Na, K, Rb, Cs, etc. Current methods involve reduction of trimethyl borate with sodium hydride. Structure In the borohydride anion and most of its modifications, boron has a tetrahedral structure. The reactivity of the B−H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name nitrite also refers to organic compounds having the –ONO group, which are esters of nitrous acid. Production Sodium nitrite is made industrially by passing a mixture of nitrogen oxides into aqueous sodium hydroxide or sodium carbonate solution: : The product is purified by recrystallization. Alkali metal nitrites are thermally stable up to and beyond their melting point (441 °C for KNO2). Ammonium nitrite can be made from dinitrogen trioxide, N2O3, which is formally the anhydride of nitrous acid: :2 NH3 + H2O + N2O3 → 2 NH4NO2 Structure The nitrite ion has a symmetrical structure (C2v molecular point group, symmetry), with both N–O bonds having equal length and a bond angle of about 115°. In valence bond theory, it is des ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted by a magnetic field. Diamagnetism is a quantum mechanical effect that occurs in all materials; when it is the only contribution to the magnetism, the material is called diamagnetic. In paramagnetic and ferromagnetic substances, the weak diamagnetic force is overcome by the attractive force of magnetic dipoles in the material. The magnetic permeability of diamagnetic materials is less than the permeability of vacuum, ''μ''0. In most materials, diamagnetism is a weak effect which can be detected only by sensitive laboratory instruments, but a superconductor acts as a strong diamagnet because it repels a magnetic field entirely from its interior. Diamagnetism was first discovered when Anton Brugmans observed in 1778 that bismuth was repel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylate
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an ion with negative charge. Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,...; ''carboxylate esters'' have the general formula (or ). R and R′ are organic groups; R′ ≠ H. Synthesis Carboxylate ions can be formed by deprotonation of carboxylic acids. Such acids typically have p''K''a of less than 5, meaning that they can be deprotonated by many bases, such as sodium hydroxide or sodium bicarbonate. :RCOOH + NaOH -> RCOONa + H2O Resonance stabilization of the carboxylate ion Carboxylic acids easily dissociate into a carboxylate anion and a positively charged hydrogen ion (proton), much more readily than alcohols do (into an alkoxide ion and a proton), because the carboxylate ion is stabilized by resonance. The negative charge that is left after deprotonation of the carboxyl group is delocalized between ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

3C5H3.png)