|
Pentaerythritol Tetrakis(3,5-di-tert-butyl-4-hydroxyhydrocinnamate)
Pentaerythritol tetrakis(3,5-di-tert-butyl-4-hydroxyhydrocinnamate) is a chemical compound composed of 4 sterically hindered phenols linked through a pentaerythritol core. It is used as primary antioxidant for stabilizing polymers, particularly polyethylene and polypropylene. Synthesis Base catalysed Michael addition of methyl acrylate to 2,6-di-tert-butylphenol forms the intermediate butyl- phloretic ester. High temperature transesterification of this with pentaerythritol gives the final product. Driving this reaction to completion can be difficult and commercial samples often contain low levels of the tri-ester. Properties The linking of phenols together with pentaerythritol maintains their activity with greatly reduced volatility. This is important during the processing and molding steps where the plastic is heated to molten, typically several hundred degrees. See also * Irganox 1098 Irganox 1098 is the trade name for ''N'',''N''′-(hexane-1,6-diyl)bis -(3,5-di-''tert''- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 billion kg/year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical drugs. Properties Phenol is an organic compound appreciably soluble in water, with about 84.2 g dissolving in 1000 mL (0.895 M). Homogeneous mixtures of phenol and water at phenol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentaerythritol
Pentaerythritol is an organic compound with the formula C(CH2OH)4. Classified as a polyol, it is a white solid. Pentaerythritol is a building block for the synthesis and production of explosives, plastics, paints, appliances, cosmetics, and many other commercial products. The word pentaerythritol is a blend of ''penta-'' in reference to its 5 carbon atoms and ''erythritol'', which also possesses 4 alcohol groups. Synthesis Pentaerythritol was first reported in 1891 by German chemist Bernhard Tollens and his student P. Wigand. It may be prepared via a base-catalyzed multiple-addition reaction between acetaldehyde and 3 equivalents of formaldehyde to give pentaerythrose (CAS: 3818-32-4), followed by a Cannizzaro reaction with a fourth equivalent of formaldehyde to give the final product. Uses Pentaerythritol is a versatile building block for the preparation of many compounds, particularly polyfunctionalized derivatives. applications include alkyd resins, varnishes, polyvinyl c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer Stabilizers
Polymer stabilizers (British: polymer stabilisers) are chemical additives which may be added to polymeric materials, such as plastics and rubbers, to inhibit or retard their degradation. Common polymer degradation processes include oxidation, UV degradation, UV-damage, Thermal degradation of polymers, thermal degradation, ozonolysis, combinations thereof such as photo-oxidation of polymers, photo-oxidation, as well as reactions with catalyst residues, dyes, or impurities. All of these degrade the polymer at a chemical level, via chain scission, uncontrolled recombination and cross-linking, which adversely affects many key properties such as strength, malleability, appearance and colour. Stabilizers are used at all stages of the polymer life-cycle. They allow plastic items to be produced faster and with fewer defects, extend their useful lifespan, and facilitate their recycling. However they also continue to stabilise waste plastic, causing it to remain in the environment for longer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
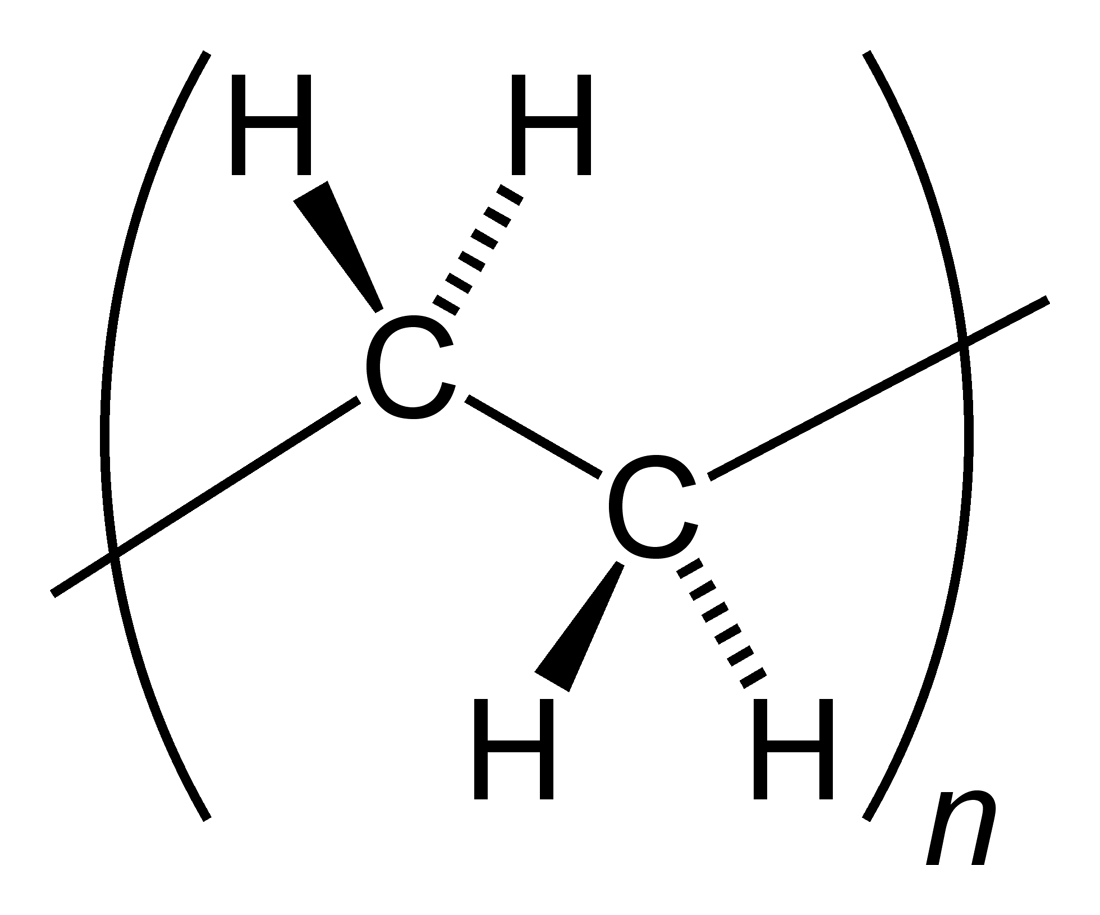
Polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging ( plastic bags, plastic films, geomembranes and containers including bottles, etc.). , over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market. Many kinds of polyethylene are known, with most having the chemical formula (C2H4)''n''. PE is usually a mixture of similar polymers of ethylene, with various values of ''n''. It can be ''low-density'' or ''high-density'': low-density polyethylene is extruded using high pressure () and high temperature (), while high-density polyethylene is extruded using low pressure () and low temperature (). Polyethylene is usually thermoplastic, but it can be modified to become thermosetting instead, for example, in cross-linked polyethylene. History Polyethylene was first synthesized by the German chemist Hans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polypropylene
Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications. It is produced via chain-growth polymerization from the monomer propylene. Polypropylene belongs to the group of polyolefins and is partially crystalline and non-polar. Its properties are similar to polyethylene, but it is slightly harder and more heat-resistant. It is a white, mechanically rugged material and has a high chemical resistance. Bio-PP is the bio-based counterpart of polypropylene (PP). Polypropylene is the second-most widely produced commodity plastic (after polyethylene). In 2019, the global market for polypropylene was worth $126.03 billion. Revenues are expected to exceed US$145 billion by 2019. The sales of this material are forecast to grow at a rate of 5.8% per year until 2021. History Phillips Petroleum chemists J. Paul Hogan and Robert Banks first demonstrated the polymerization of propylene in 1951. The stereoselective polymerization t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michael Addition
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds. The Michael addition is an important atom-economical method for diastereoselective and enantioselective C–C bond formation, and many asymmetric variants exist : In this general Michael addition scheme, either or both of R and R' on the nucleophile (the Michael donor) represent electron-withdrawing substituents such as acyl, cyano, nitro, or sulfone groups, which make the adjacent methylene hydrogen acidic enough to form a carbanion when reacted with the base, ''B:''. For the alkene (the Michael acceptor), the R" substituent is usually a carbonyl, which makes the compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Acrylate
Methyl acrylate is an organic compound, more accurately the methyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced to make acrylate fiber, which is used to weave synthetic carpets. It is also a reagent in the synthesis of various pharmaceutical intermediates. Production The standard industrial reaction for producing methyl acrylate is esterification with methanol under acid catalysis (sulfuric acid, p-toluenesulfonic acid or acidic ion exchangers.). The transesterification is facilitated because methanol and methyl acrylate form a low boiling azeotrope (boiling point 62–63 °C). The patent literature describes a one-pot route involving vapor-phase oxidation of propene or 2-propenal with oxygen in the presence of methanol. Other methods Methyl acrylate can be prepared by debromination of methyl 2,3-dibromopropanoate with zinc.F. Beilstein: ''Handbuch der organischen Chemie'', 3. Auflage, 1. Band. Verlag Leopold Voss ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,6-di-tert-butylphenol
2,6-Di-''tert''-butylphenol is an organic compound with the structural formula 2,6-((CH3)3C)2C6H3OH. This colorless solid alkylated phenol and its derivatives are used industrially as UV stabilizers and antioxidants for hydrocarbon-based products ranging from petrochemicals to plastics. Illustrative of its usefulness, it prevents gumming in aviation fuels. Production 2,6-Di-''tert''-butylphenol is prepared industrially via the Friedel–Crafts alkylation of phenol with isobutene catalyzed by aluminium phenoxide: :C6H5OH + 2 CH2=C(CH3)2 → ((CH3)3C)2C6H3OH In this way, approximately 2.5M kg/y are produced. Applications Its dominant use is as an antioxidant. 2,6-di-''tert''-butylphenol is a precursor to more complex compounds used as antioxidants and light-protection agents for the stabilization for polymers. Of particular note is methyl-3-(3,5-di-''tert''-butyl-4-hydroxyphenyl)-propionate (CAS# 6386-38-5), which is formed by the Michael addition of methyl methacrylat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phloretic Acid
Phloretic acid is an organic compound with the formula HOC6H4(CH2)2CO2H. It is a white solid. The compound contains both phenol and carboxylic acid functional groups. It is sometimes called Desaminotyrosine (DAT) because it is identical to the common alpha amino acid tyrosine except for the absence of the amino functional group on the alpha carbon. Production and occurrence Phloretic acid is produced by reduction of the unsaturated side chain of p-coumaric acid. Together with phloroglucinol, it is produced by the action of the enzyme phloretin hydrolase on phloretin. It is found in olives. It is found in the rumen of sheep fed with dried grass. It is also an urinary metabolite of tyrosine in rats. Polyesters have been prepared from phloretic acid. It is one of the products of flavonoid Flavonoids (or bioflavonoids; from the Latin word ''flavus'', meaning yellow, their color in nature) are a class of polyphenolic secondary metabolites found in plants, and thus commo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transesterification
In organic chemistry, transesterification is the process of exchanging the organic group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. The reaction can also be accomplished with the help of other enzymes, particularly lipases (one example is the lipase E.C.3.1.1.3). Strong acids catalyse the reaction by donating a proton to the carbonyl group, thus making it a more potent electrophile, whereas bases catalyse the reaction by removing a proton from the alcohol, thus making it more nucleophilic. If the alcohol produced by the reaction can be separated from the reactants by distillation this will drive the equilibrium toward the products, this means that esters with larger alkoxy groups can be made from methyl or ethyl esters in high purity by heating the mixture of ester, acid/base, and large alcohol. Mechanism In the transesterification mechanism, the carbonyl carbon of the starting e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Irganox 1098
Irganox 1098 is the trade name for ''N'',''N''′-(hexane-1,6-diyl)bis -(3,5-di-''tert''-butyl-4-hydroxyphenyl)propanamide'', a primary antioxidant manufactured by BASF primarily used for stabilizing polymers, especially polyamide A polyamide is a polymer with repeating units linked by amide bonds. Polyamides occur both naturally and artificially. Examples of naturally occurring polyamides are proteins, such as wool and silk. Artificially made polyamides can be made through ...s. It is noted for its thermal stability as well as its non-discoloring properties. See also * Pentaerythritol tetrakis(3,5-di-tert-butyl-4-hydroxyhydrocinnamate) References Phenol antioxidants {{polymer-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate is a hindered phenolic antioxidant commonly used as a polymer stabiliser. Synthesis Base catalysed Michael addition of methyl acrylate to 2,6-di-tert-butylphenol forms the intermediate butyl- phloretic ester. High temperature transesterification of this with stearyl alcohol gives the final product. Applications Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate is significantly less volatile than simpler phenolic antioxidants such as butylhydroxytoluene (BHT). This makes it more suitable to stabilising plastics, as it is not driven out by the high temperatures experienced during plastic extrusion and moulding, when they are heated to 150-320 °C (300–600 °F). It is widely used in the commodity plastics, particularly in polyethylenes and polypropylene. It has approval for use in food contact materials, such as plastic food packaging in the EU and US, amongst others. It is one of the most common p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |