|
PTX3
Pentraxin-related protein PTX3 also known as TNF-inducible gene 14 protein (TSG-14) is a protein that in humans is encoded by the ''PTX3'' gene. Pentraxin 3 (ptx3) is a member of the pentraxin superfamily. This super family characterized by cyclic multimeric structure. PTX3 is rapidly produced and released by several cell types, in particular by mononuclear phagocytes, dendritic cells (DCs), fibroblasts and endothelial cells in response to primary inflammatory signals [e.g., toll-like receptor (TLR) engagement, TNFα, Interleukin 1, IL-1β]. PTX3 binds with high affinity to the complement component C1Q complex, C1q, the extracellular matrix component TNFα induced protein 6 (TNFAIP6; also called TNF-stimulated gene 6, TSG-6) and selected microorganisms, including ''Aspergillus fumigatus'' and ''Pseudomonas aeruginosa''. PTX3 activates the classical complement pathway, classical pathway of complement activation and facilitates pathogen recognition by macrophages and DCs. Structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentraxin
Pentraxins (PTX), also known as pentaxins, are an evolutionary conserved family of proteins characterised by containing a pentraxin protein domain. Proteins of the pentraxin family are involved in acute immunological responses. They are a class of pattern recognition receptors (PRRs). They are a superfamily of multifunctional conserved proteins, some of which are components of the humoral arm of innate immunity and behave as functional ancestors of antibodies (Abs). They are known as classical acute phase proteins (APP), known for over a century. Structure Pentraxins are characterised by calcium dependent ligand binding and a distinctive flattened β-jellyroll structure similar to that of the legume lectins. The name "pentraxin" is derived from the Greek word for five (penta) and axle (axis) relating to the radial symmetry of five monomers forming a ring approximately 95Å across and 35Å deep observed in the first members of this family to be identified. The "short" pentra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serum Amyloid P Component
The serum amyloid P component (SAP) is the identical serum form of amyloid P component (AP), a 25kDa pentameric protein first identified as the pentagonal constituent of in vivo pathological deposits called "amyloid". APCS is its human gene. In amyloidosis AP makes up 14% of the dry mass of amyloid deposits and is thought to be an important contributor to the pathogenesis of a related group of diseases called the Amyloidoses. These conditions are characterised by the ordered aggregation of normal globular proteins and peptides into insoluble fibres which disrupt tissue architecture and are associated with cell death. AP is thought to decorate and stabilise aggregates by preventing proteolytic cleavage and hence inhibiting fibril removal via the normal protein scavenging mechanisms. This association is utilised in the routine clinical diagnostic technique of SAP scintigraphy whereby radio-labelled protein is injected into patients to locate areas of amyloid deposition. The SAP-a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TNFAIP6
Tumor necrosis factor-inducible gene 6 protein also known as TNF-stimulated gene 6 protein or TSG-6 is a protein that in humans is encoded by the ''TNFAIP6'' (tumor necrosis factor, alpha-induced protein 6) gene. Structure and function TSG-6 is a 30 atomic mass unit, kDa secreted protein that contains a hyaluronan-binding LINK domain a and thus is a member of the hyaluronan-binding protein family, also called hyaladherins. The hyaluronan-binding domain is known to be involved in extracellular matrix stability and cell migration. This protein has been shown to form a stable, covalent complex with inter-alpha-inhibitor (ITIH1, IαI), and thus enhance the serine protease inhibitory activity of IαI, which is important in the protease network associated with inflammation. The expression of this gene can be induced by a number of signalling molecules, principally tumor necrosis factor α (Tumor necrosis factor-alpha, TNF-α) and interleukin-1 (interleukin 1, IL-1). The expression can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Septic Shock
Septic shock is a potentially fatal medical condition that occurs when sepsis, which is organ injury or damage in response to infection, leads to dangerously low blood pressure and abnormalities in cellular metabolism. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) defines septic shock as a subset of sepsis in which particularly profound circulatory, cellular, and metabolic abnormalities are associated with a greater risk of mortality than with sepsis alone. Patients with septic shock can be clinically identified by requiring a vasopressor to maintain a mean arterial pressure of 65 mm Hg or greater and having serum lactate level greater than 2 mmol/L (>18 mg/dL) in the absence of hypovolemia. This combination is associated with hospital mortality rates greater than 40%. The primary infection is most commonly caused by bacteria, but also may be by fungi, viruses or parasites. It may be located in any part of the body, but mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acute-phase Protein
Acute-phase proteins (APPs) are a class of proteins whose concentrations in blood plasma either increase (positive acute-phase proteins) or decrease (negative acute-phase proteins) in response to inflammation. This response is called the ''acute-phase reaction'' (also called ''acute-phase response''). The acute-phase reaction characteristically involves fever, acceleration of peripheral leukocytes, circulating neutrophils and their precursors. The terms ''acute-phase protein'' and ''acute-phase reactant'' (APR) are often used synonymously, although some APRs are (strictly speaking) polypeptides rather than proteins. In response to injury, local inflammatory cells (neutrophil granulocytes and macrophages) secrete a number of cytokines into the bloodstream, most notable of which are the interleukins IL1, and IL6, and TNF-α. The liver responds by producing many acute-phase reactants. At the same time, the production of a number of other proteins is reduced; these proteins are, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Helix
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues earlier along the protein sequence. The alpha helix is also called a classic Pauling–Corey–Branson α-helix. The name 3.613-helix is also used for this type of helix, denoting the average number of residues per helical turn, with 13 atoms being involved in the ring formed by the hydrogen bond. Among types of local structure in proteins, the α-helix is the most extreme and the most predictable from sequence, as well as the most prevalent. Discovery In the early 1930s, William Astbury showed that there were drastic changes in the X-ray fiber diffraction of moist wool or hair fibers upon significant stretching. The data suggested that the unstretched fibers had a coiled molecular structure with a characteristic repeat of ≈. Astb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Sheet
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet. A β-strand is a stretch of polypeptide chain typically 3 to 10 amino acids long with backbone in an extended conformation. The supramolecular association of β-sheets has been implicated in the formation of the fibrils and protein aggregates observed in amyloidosis, notably Alzheimer's disease. History The first β-sheet structure was proposed by William Astbury in the 1930s. He proposed the idea of hydrogen bonding between the peptide bonds of parallel or antiparallel extended β-strands. However, Astbury did not have the necessary data on the bond geometry of the amino acids in order to build accurate models, especially since he did not then know that the peptide bond was planar. A refined versi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homology Modeling
Homology modeling, also known as comparative modeling of protein, refers to constructing an atomic-resolution model of the "''target''" protein from its amino acid sequence and an experimental three-dimensional structure of a related homologous protein (the "''template''"). Homology modeling relies on the identification of one or more known protein structures likely to resemble the structure of the query sequence, and on the production of an alignment that maps residues in the query sequence to residues in the template sequence. It has been seen that protein structures are more conserved than protein sequences amongst homologues, but sequences falling below a 20% sequence identity can have very different structure. Evolutionarily related proteins have similar sequences and naturally occurring homologous proteins have similar protein structure. It has been shown that three-dimensional protein structure is evolutionarily more conserved than would be expected on the basis of sequence ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their crystallographic disorder, and various other information. Since many materials can form crystals—such as salts, metals, minerals, semiconductors, as well as various inorganic, organic, and biological molecules—X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences among various mat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
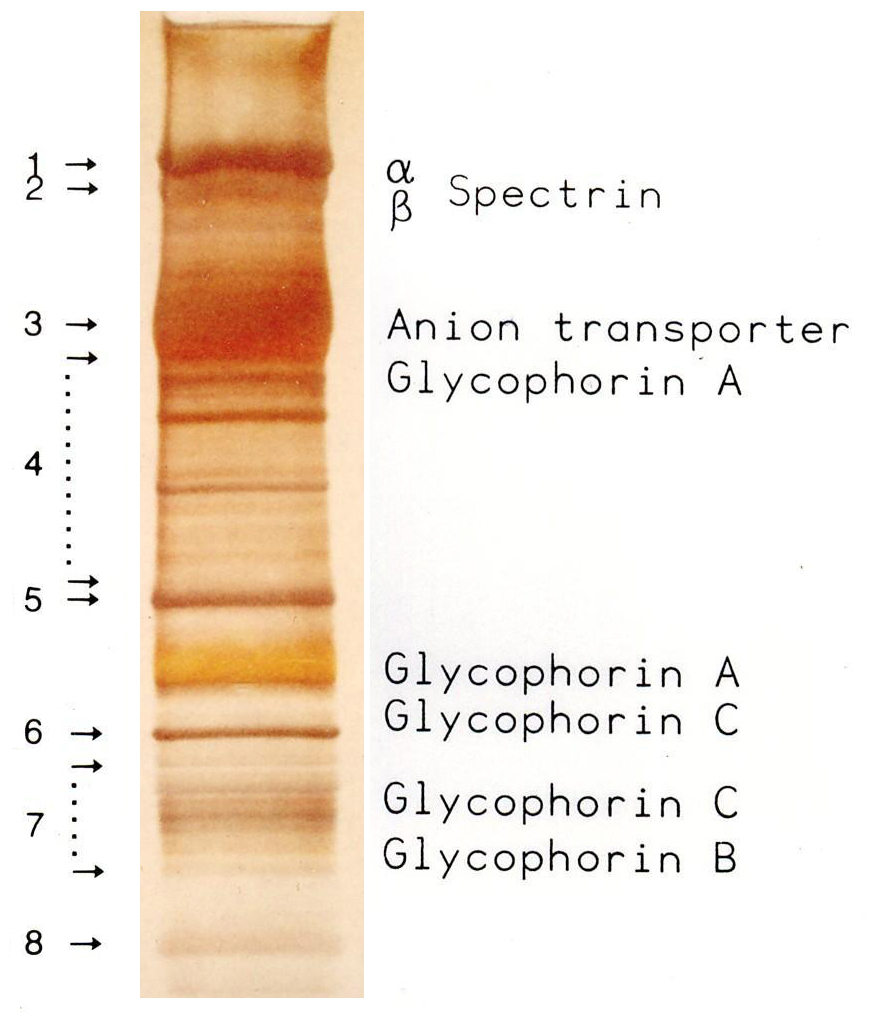
SDS-PAGE
SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) is a discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate proteins with molecular masses between 5 and 250 kDa. The combined use of sodium dodecyl sulfate (SDS, also known as sodium lauryl sulfate) and polyacrylamide gel allows to eliminate the influence of structure and charge, and proteins are separated solely on the basis of differences in their molecular weight. Properties SDS-PAGE is an electrophoresis method that allows protein separation by mass. The medium (also referred to as ′matrix′) is a polyacrylamide-based discontinuous gel. The polyacrylamide-gel is typically sandwiched between two glass plates in a slab gel. Although tube gels (in glass cylinders) were used historically, they were rapidly made obsolete with the invention of the more convenient slab gels. In addition, SDS (sodium dodecyl sulfate) is used. About 1.4 grams of S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |