SDS-PAGE on:
[Wikipedia]
[Google]
[Amazon]
 SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) is a discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate
SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) is a discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate

 SDS-PAGE is an
SDS-PAGE is an
 When using different buffers in the gel (discontinuous gel electrophoresis), the gels are made up to one day prior to electrophoresis, so that the
When using different buffers in the gel (discontinuous gel electrophoresis), the gels are made up to one day prior to electrophoresis, so that the
 During sample preparation, the sample buffer, and thus SDS, is added in excess to the proteins, and the sample is then heated to 95 °C for five minutes, or alternatively 70 °C for ten minutes. Heating disrupts the
During sample preparation, the sample buffer, and thus SDS, is added in excess to the proteins, and the sample is then heated to 95 °C for five minutes, or alternatively 70 °C for ten minutes. Heating disrupts the
 For separation, the denatured samples are loaded onto a gel of polyacrylamide, which is placed in an electrophoresis buffer with suitable electrolytes. Thereafter, a
For separation, the denatured samples are loaded onto a gel of polyacrylamide, which is placed in an electrophoresis buffer with suitable electrolytes. Thereafter, a
 At the end of the electrophoretic separation, all proteins are sorted by size and can then be analyzed by other methods, e. g. protein staining such as Coomassie staining (most common and easy to use), silver staining (highest sensitivity), stains all staining,
At the end of the electrophoretic separation, all proteins are sorted by size and can then be analyzed by other methods, e. g. protein staining such as Coomassie staining (most common and easy to use), silver staining (highest sensitivity), stains all staining,
 After protein staining and documentation of the banding pattern, the polyacrylamide gel can be dried for archival storage. Proteins can be extracted from it at a later date. The gel is either placed in a drying frame (with or without the use of heat) or in a vacuum dryer. The drying frame consists of two parts, one of which serves as a base for a wet
After protein staining and documentation of the banding pattern, the polyacrylamide gel can be dried for archival storage. Proteins can be extracted from it at a later date. The gel is either placed in a drying frame (with or without the use of heat) or in a vacuum dryer. The drying frame consists of two parts, one of which serves as a base for a wet
 For a more accurate determination of the molecular weight, the relative migration distances of the individual protein bands are measured in the separating gel. The measurements are usually performed in triplicate for increased accuracy. The relative mobility (called Rf value or Rm value) is the quotient of the distance of the band of the protein and the distance of the buffer front. The distances of the bands and the buffer front are each measured from the beginning of the separation gel. The distance of the buffer front roughly corresponds to the distance of the bromophenol blue contained in the sample buffer. The relative distances of the proteins of the size marker are plotted semi-logarithmically against their known molecular weights. By comparison with the linear part of the generated graph or by a regression analysis, the molecular weight of an unknown protein can be determined by its relative mobility.
Bands of proteins with glycosylations can be blurred. Proteins with many basic amino acids (e. g.
For a more accurate determination of the molecular weight, the relative migration distances of the individual protein bands are measured in the separating gel. The measurements are usually performed in triplicate for increased accuracy. The relative mobility (called Rf value or Rm value) is the quotient of the distance of the band of the protein and the distance of the buffer front. The distances of the bands and the buffer front are each measured from the beginning of the separation gel. The distance of the buffer front roughly corresponds to the distance of the bromophenol blue contained in the sample buffer. The relative distances of the proteins of the size marker are plotted semi-logarithmically against their known molecular weights. By comparison with the linear part of the generated graph or by a regression analysis, the molecular weight of an unknown protein can be determined by its relative mobility.
Bands of proteins with glycosylations can be blurred. Proteins with many basic amino acids (e. g.
Protocol for BisTris SDS-PAGE
at OpenWetWare.org Electrophoresis
 SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) is a discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate
SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) is a discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s with molecular masses between 5 and 250 kDa
The dalton or unified atomic mass unit (symbols: Da or u) is a non-SI unit of mass widely used in physics and chemistry. It is defined as of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at re ...
. The combined use of sodium dodecyl sulfate
Sodium dodecyl sulfate (SDS) or sodium lauryl sulfate (SLS), sometimes written sodium laurilsulfate, is an organic compound with the formula . It is an anionic surfactant used in many cleaning and hygiene products. This compound is the sodium salt ...
(SDS, also known as sodium lauryl sulfate) and polyacrylamide
Polyacrylamide (abbreviated as PAM) is a polymer with the formula (-CH2CHCONH2-). It has a linear-chain structure. PAM is highly water-absorbent, forming a soft gel when hydrated. In 2008, an estimated 750,000,000 kg were produced, mainly fo ...
gel allows to eliminate the influence of structure and charge, and proteins are separated solely on the basis of differences in their molecular weight.
Properties

electrophoresis
Electrophoresis, from Ancient Greek ἤλεκτρον (ḗlektron, "amber") and φόρησις (phórēsis, "the act of bearing"), is the motion of dispersed particles relative to a fluid under the influence of a spatially uniform electric fi ...
method that allows protein separation by mass. The medium (also referred to as ′matrix′) is a polyacrylamide-based discontinuous gel. The polyacrylamide-gel is typically sandwiched between two glass plates in a slab gel
Slab or SLAB may refer to:
Physical materials
* Concrete slab, a flat concrete plate used in construction
* Stone slab, a flat stone used in construction
* Slab (casting), a length of metal
* Slab (geology), that portion of a tectonic plate that i ...
. Although tube gels
Tube or tubes may refer to:
* ''Tube'' (2003 film), a 2003 Korean film
* ''The Tube'' (TV series), a music related TV series by Channel 4 in the United Kingdom
* "Tubes" (Peter Dale), performer on the Soccer AM television show
* Tube (band), a ...
(in glass cylinders) were used historically, they were rapidly made obsolete with the invention of the more convenient slab gels. In addition, SDS (sodium dodecyl sulfate
Sodium dodecyl sulfate (SDS) or sodium lauryl sulfate (SLS), sometimes written sodium laurilsulfate, is an organic compound with the formula . It is an anionic surfactant used in many cleaning and hygiene products. This compound is the sodium salt ...
) is used. About 1.4 grams of SDS bind to a gram of protein, corresponding to one SDS molecule per two amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
s. SDS acts as a surfactant
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsion#Emulsifiers , ...
, masking the proteins' intrinsic charge and conferring them very similar charge-to-mass ratios. The intrinsic charges of the proteins are negligible in comparison to the SDS loading, and the positive charges are also greatly reduced in the basic pH range of a separating gel. Upon application of a constant electric field, the protein migrate towards the anode, each with a different speed, depending on its mass. This simple procedure allows precise protein separation by mass.
SDS tends to form spherical micelle
A micelle () or micella () (plural micelles or micellae, respectively) is an aggregate (or supramolecular assembly) of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension (also known as associated coll ...
s in aqueous solutions above a certain concentration called the critical micellar concentration (CMC). Above the critical micellar concentration of 7 to 10 millimolar in solutions, the SDS simultaneously occurs as single molecules (monomer
In chemistry, a monomer ( ; '' mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
) and as micelles, below the CMC SDS occurs only as monomers in aqueous solutions. At the critical micellar concentration, a micelle consists of about 62 SDS molecules. However, only SDS monomers bind to proteins via hydrophobic interactions, whereas thy SDS micelles are anionic on the outside and do not adsorb any protein. SDS is amphipathic in nature, which allows it to unfold both polar and nonpolar sections of protein structure. In SDS concentrations above 0.1 millimolar, the unfolding of proteins begins, and above 1 mM, most proteins are denatured. Due to the strong denaturing effect of SDS and the subsequent dissociation of protein complexes, quaternary structure
Protein quaternary structure is the fourth (and highest) classification level of protein structure. Protein quaternary structure refers to the structure of proteins which are themselves composed of two or more smaller protein chains (also refe ...
s can generally not be determined with SDS. Exceptions are proteins that are stabilised by covalent cross-link
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
ing e.g. -S-S- linkages and the SDS-resistant protein complexes, which are stable even in the presence of SDS (the latter, however, only at room temperature). To denature the SDS-resistant complexes a high activation energy is required, which is achieved by heating. SDS resistance is based on a metastability of the protein fold. Although the native, fully folded, SDS-resistant protein does not have sufficient stability in the presence of SDS, the chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the ...
of denaturation at room temperature occurs slowly. Stable protein complexes are characterised not only by SDS resistance but also by stability against protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the ...
s and an increased biological half-life
Biological half-life (also known as elimination half-life, pharmacologic half-life) is the time taken for concentration of a biological substance (such as a medication) to decrease from its maximum concentration ( Cmax) to half of Cmax in the bl ...
.
Alternatively, polyacrylamide gel electrophoresis can also be performed with the cationic surfactants CTAB in a CTAB-PAGE, or 16-BAC in a BAC-PAGE.
Procedure
The SDS-PAGE method is composed of gel preparation, sample preparation, electrophoresis, protein staining orwestern blot
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detect ...
ting and analysis of the generated banding pattern.
Gel production

diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical ...
does not lead to a mixing of the buffers. The gel is produced by free radical polymerization
In polymer chemistry, free-radical polymerization (FRP) is a method of polymerization by which a polymer forms by the successive addition of free-radical building blocks ( repeat units). Free radicals can be formed by a number of different mechani ...
in a mold consisting of two sealed glass plates with spacers between the glass plates. In a typical mini-gel setting, the spacers have a thickness of 0.75 mm or 1.5 mm, which determines the loading capacity of the gel. For pouring the gel solution, the plates are usually clamped in a stand which temporarily seals the otherwise open underside of the glass plates with the two spacers. For the gel solution, acrylamide is mixed as gel-former (usually 4% V/V in the stacking gel and 10-12 % in the separating gel), methylenebisacrylamide as a cross-linker, stacking or separating gel buffer, water and SDS. By adding the catalyst TEMED and the radical initiator ammonium persulfate (APS) the polymerisation is started. The solution is then poured between the glass plates without creating bubbles. Depending on the amount of catalyst and radical starter and depending on the temperature, the polymerisation lasts between a quarter of an hour and several hours. The lower gel (separating gel) is poured first and covered with a few drops of a barely water-soluble alcohol (usually buffer-saturated butanol or isopropanol), which eliminates bubbles from the meniscus and protects the gel solution of the radical scavenger oxygen. After the polymerisation of the separating gel, the alcohol is discarded and the residual alcohol is removed with filter paper Filter paper is a semi-permeable paper barrier placed perpendicular to a liquid or air flow. It is used to separate fine solid particles from liquids or gases.
The raw materials are different Pulp (paper), paper pulps. The pulp may be made from soft ...
. After addition of APS and TEMED to the stacking gel solution, it is poured on top of the solid separation gel. Afterwards, a suitable sample comb is inserted between the glass plates without creating bubbles. The sample comb is carefully pulled out after polymerisation, leaving pockets for the sample application. For later use of proteins for protein sequencing
Protein sequencing is the practical process of determining the amino acid sequence of all or part of a protein or peptide. This may serve to identify the protein or characterize its post-translational modifications. Typically, partial sequencing o ...
, the gels are often prepared the day before electrophoresis to reduce reactions of unpolymerised acrylamide with cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, some ...
s in proteins.
By using a gradient mixer, gradient gels with a gradient of acrylamide (usually from 4 to 12%) can be cast, which have a larger separation range of the molecular masses. Commercial gel systems (so-called ''pre-cast gels'') usually use the buffer substance Bis-tris methane with a pH value between 6.4 and 7.2 both in the stacking gel and in the separating gel. These gels are delivered cast and ready-to-use. Since they use only one buffer ( continuous gel electrophoresis) and have a nearly neutral pH, they can be stored for several weeks. The more neutral pH slows the hydrolysis and thus the decomposition of the polyacrylamide. Furthermore, there are fewer acrylamide-modified cysteines in the proteins. Due to the constant pH in collecting and separating gel there is no stacking effect. Proteins in BisTris gels can not be stained with ruthenium complexes. This gel system has a comparatively large separation range, which can be varied by using MES or MOPS
MOPS (3-(''N''-morpholino)propanesulfonic acid) is a buffer introduced in the 1960s, one of the twenty Good's buffers. It is a structural analog to MES, and like MES, its structure contains a morpholine ring. HEPES is a similar pH buffering ...
in the running buffer.
Sample preparation
 During sample preparation, the sample buffer, and thus SDS, is added in excess to the proteins, and the sample is then heated to 95 °C for five minutes, or alternatively 70 °C for ten minutes. Heating disrupts the
During sample preparation, the sample buffer, and thus SDS, is added in excess to the proteins, and the sample is then heated to 95 °C for five minutes, or alternatively 70 °C for ten minutes. Heating disrupts the secondary
Secondary may refer to: Science and nature
* Secondary emission, of particles
** Secondary electrons, electrons generated as ionization products
* The secondary winding, or the electrical or electronic circuit connected to the secondary winding i ...
and tertiary structure
Protein tertiary structure is the three dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains may i ...
s of the protein by disrupting hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
s and stretching the molecules. Optionally, disulfide bridge
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
s can be cleaved by reduction. For this purpose, reducing thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ...
s such as β-mercaptoethanol (β-ME, 5% by volume), dithiothreitol (DTT, 10 millimolar) or dithioerythritol
Dithioerythritol (DTE) is a sulfur containing sugar derived from the corresponding 4-carbon monosaccharide erythrose. It is an epimer of dithiothreitol (DTT). The molecular formula for DTE is C4H10O2S2.
Like DTT, DTE makes an excellent reduci ...
(DTE, 10 millimolar) are added to the sample buffer. After cooling to room temperature, each sample is pipetted into its own well in the gel, which was previously immersed in electrophoresis buffer in the electrophoresis apparatus.
In addition to the samples, a molecular-weight size marker is usually loaded onto the gel. This consists of proteins of known sizes and thereby allows the estimation (with an error of ± 10%) of the sizes of the proteins in the actual samples, which migrate in parallel in different tracks of the gel. The size marker is often pipetted into the first or last pocket of a gel.
Electrophoresis
 For separation, the denatured samples are loaded onto a gel of polyacrylamide, which is placed in an electrophoresis buffer with suitable electrolytes. Thereafter, a
For separation, the denatured samples are loaded onto a gel of polyacrylamide, which is placed in an electrophoresis buffer with suitable electrolytes. Thereafter, a voltage
Voltage, also known as electric pressure, electric tension, or (electric) potential difference, is the difference in electric potential between two points. In a static electric field, it corresponds to the work needed per unit of charge to ...
(usually around 100 V, 10-20 V per cm gel length) is applied, which causes a migration of negatively charged molecules through the gel in the direction of the positively charged anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic is ...
. The gel acts like a sieve. Small proteins migrate relatively easily through the mesh of the gel, while larger proteins are more likely to be retained and thereby migrate more slowly through the gel, thereby allowing proteins to be separated by molecular size. The electrophoresis lasts between half an hour to several hours depending on the voltage and length of gel used.
The fastest-migrating proteins (with a molecular weight of less than 5 kDa) form the buffer front together with the anionic components of the electrophoresis buffer, which also migrate through the gel. The area of the buffer front is made visible by adding the comparatively small, anionic dye bromophenol blue
Bromophenol blue (3′,3″,5′,5″-tetrabromophenolsulfonphthalein, BPB), albutest is used as a pH indicator, an electrophoretic color marker, and a dye. It can be prepared by slowly adding excess bromine to a hot solution of phenolsulfonp ...
to the sample buffer. Due to the relatively small molecule size of bromophenol blue, it migrates faster than proteins. By optical control of the migrating colored band, the electrophoresis can be stopped before the dye and also the samples have completely migrated through the gel and leave it.
The most commonly used method is the discontinuous SDS-PAGE. In this method, the proteins migrate first into a collecting gel with neutral pH, in which they are concentrated and then they migrate into a separating gel with basic pH, in which the actual separation takes place. Stacking and separating gels differ by different pore size (4-6 % T and 10-20 % T), ionic strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such a ...
and pH value
In chemistry, pH (), historically denoting "potential of hydrogen" (or "power of hydrogen"), is a scale used to specify the acidity or basicity of an aqueous solution. Acidic solutions (solutions with higher concentrations of ions) are me ...
s (pH 6.8 or pH 8.8). The electrolyte most frequently used is an SDS-containing Tris
Tris, or tris(hydroxymethyl)aminomethane, or known during medical use as tromethamine or THAM, is an organic compound with the formula (HOCH2)3CNH2, one of the twenty Good's buffers. It is extensively used in biochemistry and molecular biology as ...
-glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid ( carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinog ...
-chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride s ...
buffer system. At neutral pH, glycine predominantly forms the zwitterionic form, at high pH the glycines lose positive charges and become predominantly anionic. In the collection gel, the smaller, negatively charged chloride ions migrate in front of the proteins (as leading ions) and the slightly larger, negatively and partially positively charged glycinate ions migrate behind the proteins (as initial trailing ions), whereas in the comparatively basic separating gel both ions migrate in front of the proteins. The pH gradient between the stacking and separation gel buffers leads to a stacking effect at the border of the stacking gel to the separation gel, since the glycinate partially loses its slowing positive charges as the pH increases and then, as the former trailing ion, overtakes the proteins and becomes a leading ion, which causes the bands of the different proteins (visible after a staining) to become narrower and sharper - the stacking effect. For the separation of smaller proteins and peptides, the TRIS- Tricine buffer system of Schägger and von Jagow is used due to the higher spread of the proteins in the range of 0.5 to 50 kDa.
Gel staining
 At the end of the electrophoretic separation, all proteins are sorted by size and can then be analyzed by other methods, e. g. protein staining such as Coomassie staining (most common and easy to use), silver staining (highest sensitivity), stains all staining,
At the end of the electrophoretic separation, all proteins are sorted by size and can then be analyzed by other methods, e. g. protein staining such as Coomassie staining (most common and easy to use), silver staining (highest sensitivity), stains all staining, Amido black 10B
Amido black 10B is an amino acid staining azo dye used in biochemical research to stain for total protein on transferred membrane blots, such as the western blot. It is also used in criminal investigations to detect blood present with latent fing ...
staining, Fast green FCF staining, fluorescent stains such as epicocconone
Epicocconone is a long Stokes' shift fluorescent dye found in the fungus '' Epicoccum nigrum''. Though weakly fluorescent in water (green emission, 520 nm) it reacts reversibly with proteins to yield a product with a strong orange-red emiss ...
stain and SYPRO orange stain, and immunological detection such as the Western Blot
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detect ...
. The fluorescent dyes have a comparatively higher linearity between protein quantity and color intensity of about three orders of magnitude above the detection limit
The limit of detection (LOD or LoD) is the lowest signal, or the lowest corresponding quantity to be determined (or extracted) from the signal, that can be observed with a sufficient degree of confidence or statistical significance. However, the ...
, i. e. the amount of protein can be estimated by color intensity. When using the fluorescent protein dye trichloroethanol
2,2,2-Trichloroethanol is the chemical compound with formula . Its molecule can be described as that of ethanol, with the three hydrogen atoms at position 2 (the methyl group) replaced by chlorine atoms. It is a clear flammable liquid at room tem ...
, a subsequent protein staining is omitted if it was added to the gel solution and the gel was irradiated with UV light after electrophoresis.
In Coomassie staining, gel is fixed in a 50% ethanol 10% glacial acetic acid solution for 1 hr. Then the solution is changed for fresh one and after 1 to 12 hrs gel is changed to a staining solution (50% methanol, 10% glacial acetic acid, 0.1% coomassie brilliant blue) followed by destaining changing several times a destaining solution of 40% methanol, 10% glacial acetic acid.
Analysis
Protein staining in the gel creates a documentable banding pattern of the various proteins. *Glycoprotein
Glycoproteins are proteins which contain oligosaccharide chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glyco ...
s have differential levels of glycosylations and adsorb SDS more unevenly at the glycosylations, resulting in broader and blurred bands.
* Membrane protein
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane ...
s, because of their transmembrane domain
A transmembrane domain (TMD) is a membrane-spanning protein domain. TMDs generally adopt an alpha helix topological conformation, although some TMDs such as those in porins can adopt a different conformation. Because the interior of the lipid bi ...
, are often composed of the more hydrophobic amino acids, have lower solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubi ...
in aqueous solutions, tend to bind lipid
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids in ...
s, and tend to precipitate in aqueous solutions due to hydrophobic effect
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and exclude water molecules. The word hydrophobic literally means "water-fearing", and it describes the segregation of water and nonpolar ...
s when sufficient amounts of detergent are not present. This precipitation manifests itself for membrane proteins in a SDS-PAGE in "tailing" above the band of the transmembrane protein. In this case, more SDS can be used (by using more or more concentrated sample buffer) and the amount of protein in the sample application can be reduced.
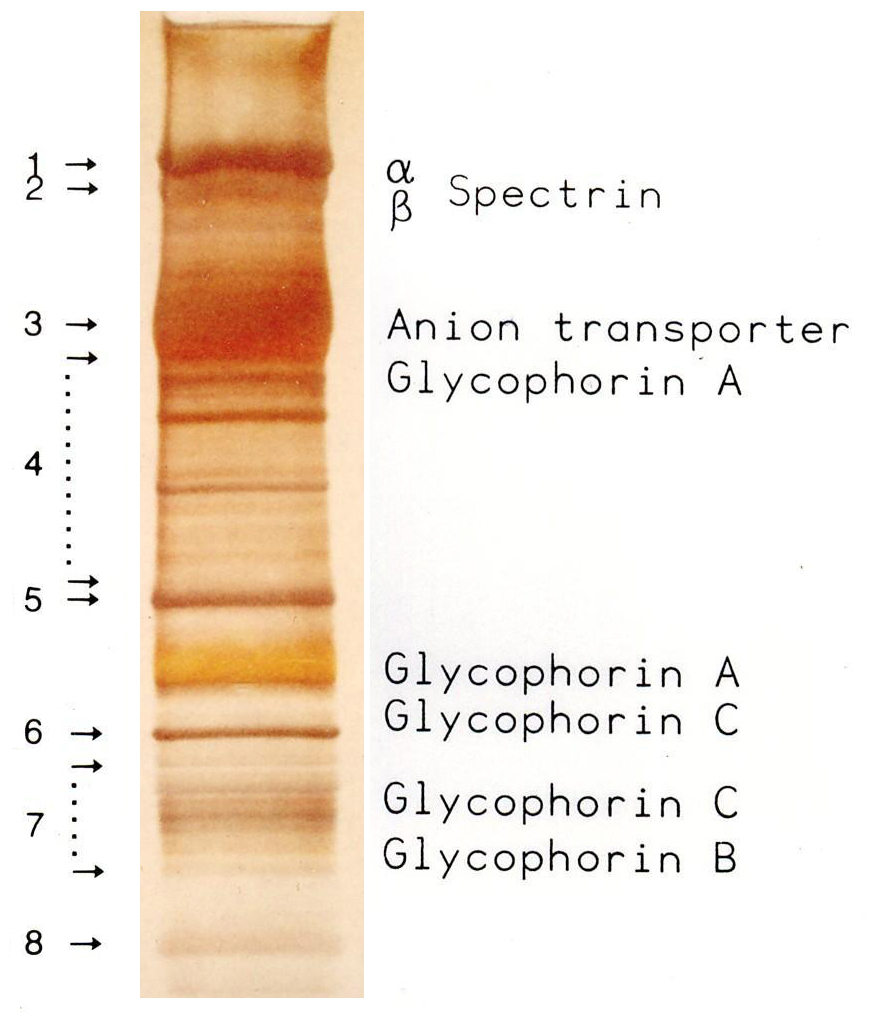
* An overloading of the gel with a soluble protein creates a semicircular band of this protein (e. g. in the marker lane of the image at 66 kDa), allowing other proteins with similar molecular weights to be covered.
* A low contrast (as in the marker lane of the image) between bands within a lane indicates either the presence of many proteins (low purity) or, if using purified proteins and a low contrast occurs only below one band, it indicates a proteolytic degradation of the protein, which first causes degradation bands, and after further degradation produces a homogeneous color ("smear") below a band.
The documentation of the banding pattern is usually done by photographing or scanning. For a subsequent recovery of the molecules in individual bands, a gel extraction can be performed.
Archiving
 After protein staining and documentation of the banding pattern, the polyacrylamide gel can be dried for archival storage. Proteins can be extracted from it at a later date. The gel is either placed in a drying frame (with or without the use of heat) or in a vacuum dryer. The drying frame consists of two parts, one of which serves as a base for a wet
After protein staining and documentation of the banding pattern, the polyacrylamide gel can be dried for archival storage. Proteins can be extracted from it at a later date. The gel is either placed in a drying frame (with or without the use of heat) or in a vacuum dryer. The drying frame consists of two parts, one of which serves as a base for a wet cellophane
Cellophane is a thin, transparent sheet made of regenerated cellulose. Its low permeability to air, oils, greases, bacteria, and liquid water makes it useful for food packaging. Cellophane is highly permeable to water vapour, but may be coated ...
film to which the gel and a one percent glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known ...
solution are added. Then a second wet cellophane film is applied bubble-free, the second frame part is put on top and the frame is sealed with clips. The removal of the air bubbles avoids a fragmentation of the gel during drying. The water evaporates through the cellophane film. In contrast to the drying frame, a vacuum dryer generates a vacuum and heats the gel to about 50 °C.
Molecular mass determination
 For a more accurate determination of the molecular weight, the relative migration distances of the individual protein bands are measured in the separating gel. The measurements are usually performed in triplicate for increased accuracy. The relative mobility (called Rf value or Rm value) is the quotient of the distance of the band of the protein and the distance of the buffer front. The distances of the bands and the buffer front are each measured from the beginning of the separation gel. The distance of the buffer front roughly corresponds to the distance of the bromophenol blue contained in the sample buffer. The relative distances of the proteins of the size marker are plotted semi-logarithmically against their known molecular weights. By comparison with the linear part of the generated graph or by a regression analysis, the molecular weight of an unknown protein can be determined by its relative mobility.
Bands of proteins with glycosylations can be blurred. Proteins with many basic amino acids (e. g.
For a more accurate determination of the molecular weight, the relative migration distances of the individual protein bands are measured in the separating gel. The measurements are usually performed in triplicate for increased accuracy. The relative mobility (called Rf value or Rm value) is the quotient of the distance of the band of the protein and the distance of the buffer front. The distances of the bands and the buffer front are each measured from the beginning of the separation gel. The distance of the buffer front roughly corresponds to the distance of the bromophenol blue contained in the sample buffer. The relative distances of the proteins of the size marker are plotted semi-logarithmically against their known molecular weights. By comparison with the linear part of the generated graph or by a regression analysis, the molecular weight of an unknown protein can be determined by its relative mobility.
Bands of proteins with glycosylations can be blurred. Proteins with many basic amino acids (e. g. histone
In biology, histones are highly basic proteins abundant in lysine and arginine residues that are found in eukaryotic cell nuclei. They act as spools around which DNA winds to create structural units called nucleosomes. Nucleosomes in turn a ...
s) can lead to an overestimation of the molecular weight or even not migrate into the gel at all, because they move slower in the electrophoresis due to the positive charges or even to the opposite direction. Accordingly, many acidic amino acids can lead to accelerated migration of a protein and an underestimation of its molecular mass.
Applications
The SDS-PAGE in combination with a protein stain is widely used in biochemistry for the quick and exact separation and subsequent analysis of proteins. It has comparatively low instrument and reagent costs and is an easy-to-use method. Because of its lowscalability
Scalability is the property of a system to handle a growing amount of work by adding resources to the system.
In an economic context, a scalable business model implies that a company can increase sales given increased resources. For example, a ...
, it is mostly used for analytical purposes and less for preparative purposes, especially when larger amounts of a protein are to be isolated.
Additionally, SDS-PAGE is used in combination with the western blot
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detect ...
for the determination of the presence of a specific protein in a mixture of proteins - or for the analysis of post-translational modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribo ...
s. Post-translational modifications of proteins can lead to a different relative mobility (i.e. a ''band shift'') or to a change in the binding of a detection antibody used in the western blot (i.e. a band disappears or appears).
In mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is u ...
of proteins, SDS-PAGE is a widely used method for sample preparation prior to spectrometry, mostly using in-gel digestion. In regards to determining the molecular mass of a protein, the SDS-PAGE is a bit more exact than an analytical ultracentrifugation, but less exact than a mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is u ...
or - ignoring post-translational modifications - a calculation of the protein molecular mass from the DNA sequence
DNA sequencing is the process of determining the nucleic acid sequence – the order of nucleotides in DNA. It includes any method or technology that is used to determine the order of the four bases: adenine, guanine, cytosine, and thymine. T ...
.
In medical diagnostics, SDS-PAGE is used as part of the HIV test and to evaluate proteinuria
Proteinuria is the presence of excess proteins in the urine. In healthy persons, urine contains very little protein; an excess is suggestive of illness. Excess protein in the urine often causes the urine to become foamy (although this symptom ma ...
. In the HIV test, HIV proteins are separated by SDS-PAGE and subsequently detected by Western Blot with HIV-specific antibodies
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of ...
of the patient, if they are present in his blood serum
Serum () is the fluid and solute component of blood which does not play a role in clotting. It may be defined as blood plasma without the clotting factors, or as blood with all cells and clotting factors removed. Serum includes all proteins not ...
. SDS-PAGE for proteinuria evaluates the levels of various serum proteins in the urine, e.g. Albumin
Albumin is a family of globular proteins, the most common of which are the serum albumins. All the proteins of the albumin family are water- soluble, moderately soluble in concentrated salt solutions, and experience heat denaturation. Album ...
, Alpha-2-macroglobulin
α2-Macroglobulin (α2M), or alpha-2-macroglobulin, is a large (720 KDa) plasma protein found in the blood. It is mainly produced by the liver, and also locally synthesized by macrophages, fibroblasts, and adrenocortical cells. In humans it is ...
and IgG
Immunoglobulin G (Ig G) is a type of antibody. Representing approximately 75% of serum antibodies in humans, IgG is the most common type of antibody found in blood circulation. IgG molecules are created and released by plasma B cells. Each IgG an ...
.
Variants
SDS-PAGE is the most widely used method for gel electrophoretic separation of proteins.Two-dimensional gel electrophoresis
Two-dimensional gel electrophoresis, abbreviated as 2-DE or 2-D electrophoresis, is a form of gel electrophoresis commonly used to analyze proteins. Mixtures of proteins are separated by two properties in two dimensions on 2D gels. 2-DE was first ...
sequentially combines isoelectric focusing or BAC-PAGE with a SDS-PAGE. Native PAGE is used if native protein folding is to be maintained. For separation of membrane proteins, BAC-PAGE or CTAB-PAGE may be used as an alternative to SDS-PAGE. For electrophoretic separation of larger protein complexes, agarose gel electrophoresis
Agarose gel electrophoresis is a method of gel electrophoresis used in biochemistry, molecular biology, genetics, and clinical chemistry to separate a mixed population of macromolecules such as DNA or proteins in a matrix of agarose, one of the ...
can be used, e.g. the SDD-AGE
In biochemistry and molecular biology, SDD-AGE is short for Semi-Denaturating Detergent Agarose Gel Electrophoresis. This is a method for detecting and characterizing large protein polymers which are stable in 2% SDS at room temperature, unlike ...
. Some enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
s can be detected via their enzyme activity
Enzyme assays are laboratory methods for measuring enzymatic activity. They are vital for the study of enzyme kinetics and enzyme inhibition.
Enzyme units
The quantity or concentration of an enzyme can be expressed in molar amounts, as with a ...
by zymography.
Alternatives
While being one of the more precise and low-cost protein separation and analysis methods, the SDS-PAGE denatures proteins. Where non-denaturing conditions are necessary, proteins are separated by a native PAGE or differentchromatographic
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a ...
methods with subsequent photometric quantification, for example affinity chromatography (or even tandem affinity purification Tandem affinity purification (TAP) is an immunoprecipitation-based purification technique for studying protein–protein interactions. The goal is to extract from a cell only the protein of interest, in complex with any other proteins it interacte ...
), size exclusion chromatography
Size-exclusion chromatography (SEC), also known as molecular sieve chromatography, is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecules ...
, ion exchange chromatography
Ion chromatography (or ion-exchange chromatography) separates ions and polar molecules based on their affinity to the ion exchanger. It works on almost any kind of charged molecule—including large proteins, small nucleotides, and amino acid ...
. Proteins can also be separated by size in a tangential flow filtration or an ultrafiltration
Ultrafiltration (UF) is a variety of membrane filtration in which forces such as pressure or concentration gradients lead to a separation through a semipermeable membrane. Suspended solids and solutes of high molecular weight are retained in the ...
. Single proteins can be isolated from a mixture by affinity chromatography or by a pull-down assay. Some historically early and cost effective but crude separation methods usually based upon a series of extractions and precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls under gravitational pull from clouds. The main forms of precipitation include drizzle, rain, sleet, snow, ice pellets, graupel and hail. ...
s using kosmotropic molecules, for example the ammonium sulfate precipitation Ammonium sulfate precipitation is one of the most commonly used methods for large and laboratory scale protein purification and fractionation that can be used to separate proteins by altering their solubility in the presence of a high salt concentra ...
and the polyethyleneglycol precipitation.
History
In 1948, Arne Tiselius was awarded theNobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
for the discovery of the principle of electrophoresis as the migration of charged and dissolved atoms or molecules in an electric field. The use of a solid matrix (initially paper discs) in a zone electrophoresis improved the separation. The discontinuous electrophoresis of 1964 by L. Ornstein and B. J. Davis made it possible to improve the separation by the stacking effect. The use of cross-linked polyacrylamide hydrogels, in contrast to the previously used paper discs or starch gels, provided a higher stability of the gel and no microbial decomposition. The denaturing effect of SDS in continuous polyacrylamide gels and the consequent improvement in resolution was first described in 1965 by David F. Summers in the working group of James E. Darnell to separate poliovirus proteins. The current variant of the SDS-PAGE was described in 1970 by Ulrich K. Laemmli and initially used to characterise the proteins in the head of bacteriophage T4. This Laemmli paper is widely cited for the invention of modern SDS-PAGE, but the technique was actually invented by Jake Maizel, who was doing a sabbatical in the MRC laboratory when Laemmli joined the lab as a postdoctoral fellow. Maizel shared his prior technology with Laemmli and together they made further improvements. Laemmli and Maizel had planned to follow up with a Methods paper but this never materialized. Maizel recounts the history of development of SDS-PAGE in brief commentary.
References
{{ReflistExternal links
Protocol for BisTris SDS-PAGE
at OpenWetWare.org Electrophoresis