|
Process Heat
Process heat refers to the application of heat during industrial processes. Some form of process heat is used during the manufacture of many common products, from concrete to glass to steel to paper. Where byproducts or wastes of the overall industrial process are available, those are often used to provide process heat. Examples include black liquor in papermaking or bagasse in sugarcane processing. Requirements The required temperature of the process varies widely, with about half the industrial process heat having operating temperatures above . These higher-temperature processes can generally only be supplied by dedicated supplies like natural gas or coal, although pre-heating from other sources is also common in order to reduce fuel use. Those processes operating below the median can draw on a much wider variety of sources, including waste heat from other processes in the same industrial process. Resistive heating would in theory be a possible source of process heat but even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is also often used to refer to the thermal energy contained in a system as a component of its internal energy and that is reflected in the temperature of the system. For both uses of the term, heat is a form of energy. An example of formal vs. informal usage may be obtained from the right-hand photo, in which the metal bar is "conducting heat" from its hot end to its cold end, but if the metal bar is considered a thermodynamic system, then the energy flowing within the metal bar is called internal energy, not heat. The hot metal bar is also transferring heat to its surroundings, a correct statement for both the strict and loose meanings of ''heat''. Another example of informal usage is the term '' heat content'', used despite the fact tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Pump
A heat pump is a device that can heat a building (or part of a building) by transferring thermal energy from the outside using a refrigeration cycle. Many heat pumps can also operate in the opposite direction, cooling the building by removing heat from the enclosed space and rejecting it outside. Units that only provide cooling are called air conditioners. When in heating mode, a refrigerant at outside temperature is being compressed. As a result, the refrigerant becomes hot. This thermal energy can be transferred to an indoor unit. After being moved outdoors again, the refrigerant is decompressed — evaporated. It has lost some of its thermal energy and returns colder than the environment. It can now take up the surrounding energy from the air or from the ground before the process repeats. Compressors, fans, and pumps run with electric energy. Common types are air-source heat pumps, ground-source heat pumps, water-source heat pumps and exhaust air heat pumps. They are a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concentrated Solar Power
Concentrated solar power (CSP, also known as concentrating solar power, concentrated solar thermal) systems generate solar power by using mirrors or lenses to concentrate a large area of sunlight into a receiver. Electricity is generated when the concentrated light is converted to heat ( solar thermal energy), which drives a heat engine (usually a steam turbine) connected to an electrical power generator or powers a thermochemical reaction. CSP had a global total installed capacity of 6,800 MW in 2021, up from 354 MW in 2005. Spain accounted for almost one third of the world's capacity, at 2,300 MW, despite no new capacity entering commercial operation in the country since 2013. The United States follows with 1,740 MW. Interest is also notable in North Africa and the Middle East, as well as China and India. The global market was initially dominated by parabolic-trough plants, which accounted for 90% of CSP plants at one point. Since about 2010, centra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomass
Biomass is plant-based material used as a fuel for heat or electricity production. It can be in the form of wood, wood residues, energy crops, agricultural residues, and waste from industry, farms, and households. Some people use the terms biomass and biofuel interchangeably, while others consider biofuel to be a ''liquid'' or ''gaseous'' fuel used for transportation, as defined by government authorities in the US and EU. The European Union's Joint Research Centre defines solid biofuel as raw or processed organic matter of biological origin used for energy, such as firewood, wood chips, and wood pellets. In 2019, biomass was used to produce 57 EJ (exajoules) of energy, compared to 190 EJ from crude oil, 168 EJ from coal, 144 EJ from natural gas, 30 EJ from nuclear, 15 EJ from hydropower, hydro and 13 EJ from wind power, wind, solar power, solar and geothermal energy, geothermal combined. Approximately 86% of modern bioenergy is used for heating applications, with 9% used for tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Waste Tires
Tire recycling, or rubber recycling, is the process of recycling waste tires that are no longer suitable for use on vehicles due to wear or irreparable damage. These tires are a challenging source of waste, due to the large volume produced, the durability of the tires, and the components in the tire that are ecologically problematic. Because tires are highly durable and non-biodegradable, they can consume valuable space in landfills. If waste tires are improperly managed they may cause rubber pollution. In 1990, it was estimated that over 1 billion scrap tires were in stockpiles in the United States. As of 2015, only 67 million tires remain in stockpiles. From 1994 to 2010, the European Union increased the amount of tires recycled from 25% of annual discards to nearly 95%, with roughly half of the end-of-life tires used for energy, mostly in cement manufacturing. Pyrolysis and devulcanization could facilitate recycling. Aside from use as fuel, the main end use for tires remai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Neutral
Carbon neutrality is a state of net-zero carbon dioxide emissions. This can be achieved by balancing emissions of carbon dioxide with its removal (often through carbon offsetting) or by eliminating emissions from society (the transition to the "post-carbon economy"). The term is used in the context of carbon dioxide-releasing processes associated with transportation, energy production, agriculture, and industry. Although the term "carbon neutral" is used, a carbon footprint also includes other greenhouse gases, measured in terms of their carbon dioxide equivalence. The term climate-neutral reflects the broader inclusiveness of other greenhouse gases in climate change, even if CO2 is the most abundant. The term "net zero" is increasingly used to describe a broader and more comprehensive commitment to decarbonization and climate action, moving beyond carbon neutrality by including more activities under the scope of indirect emissions, and often including a science-based target o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Principle Of Le Chatelier
Le Chatelier's principle (pronounced or ), also called Chatelier's principle (or the Equilibrium Law), is a principle of chemistry used to predict the effect of a change in conditions on chemical equilibria. The principle is named after French chemist Henry Louis Le Chatelier, and sometimes also credited to Karl Ferdinand Braun, who discovered it independently. It can be stated as: Phenomena in apparent contradiction to Le Chatelier's principle can also arise in systems of simultaneous equilibrium (see response reactions). Le Chatelier's principle is sometimes alluded to in discussions of topics other than thermodynamics. Thermodynamic statement The Le Chatelier–Braun principle analyzes the qualitative behaviour of a thermodynamic system when a designated one of its externally controlled state variables, say L, changes by an amount \Delta L, the 'driving change', causing a change \delta_ M, the 'response of prime interest', in its conjugate state variable M, all other ext ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
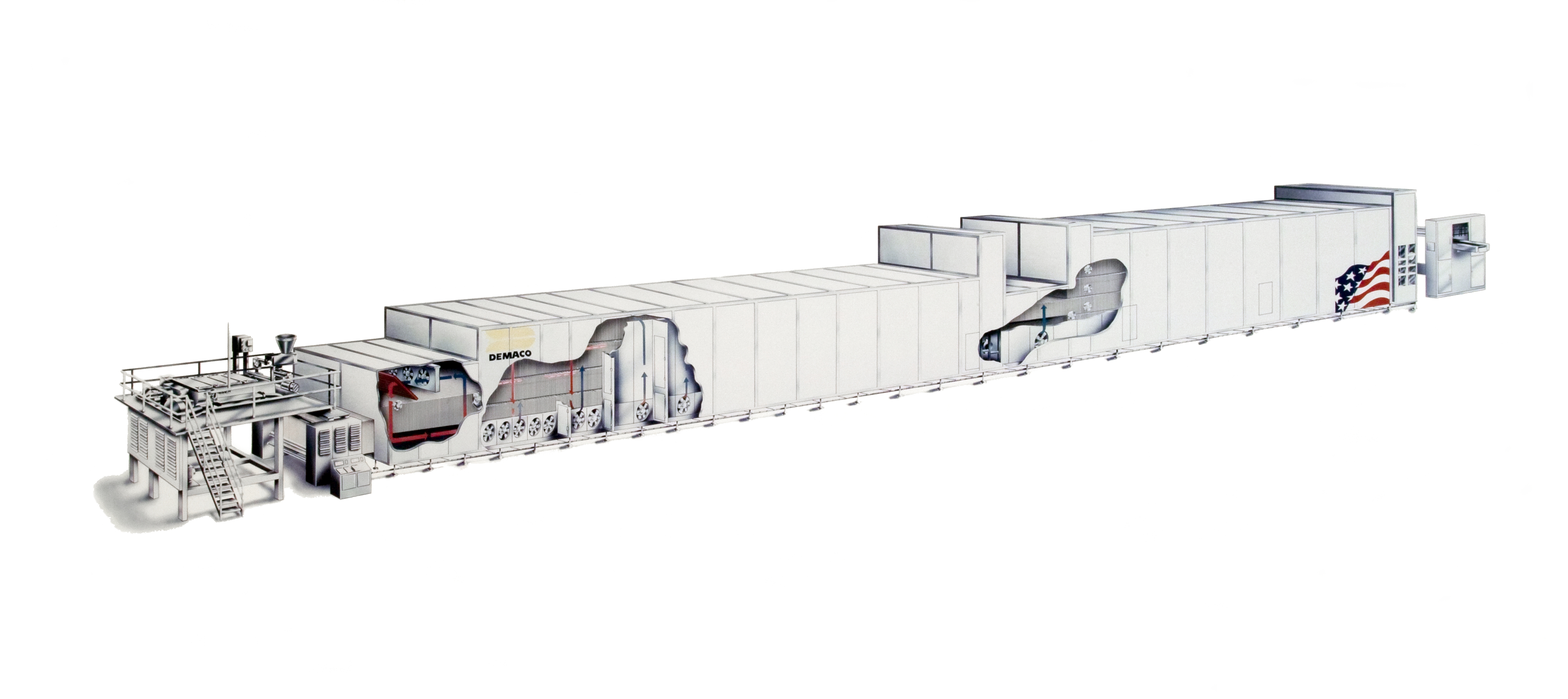
Continuous Process
Continuous production is a flow production method used to manufacture, produce, or process materials without interruption. Continuous production is called a continuous process or a continuous flow process because the materials, either dry bulk or fluids that are being processed are continuously in motion, undergoing chemical reactions or subject to mechanical or heat treatment. Continuous processing is contrasted with batch production. Continuous usually means operating 24 hours per day, seven days per week with infrequent maintenance shutdowns, such as semi-annual or annual. Some chemical plants can operate for more than one to two years without a shutdown. Blast furnaces can run from four to ten years without stopping. Common processes Some common continuous processes are the following: * Oil refining *Chemicals *Synthetic fibers *Fertilizers * Pulp and paper *Blast furnace (iron) *Metal smelting *Power stations *Natural gas processing *Sanitary waste water treatment *Co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system. This state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium. Historical introduction The concept of chemical equilibrium was developed in 1803, after Berthollet found that some chemical reactions are reversible. For any reaction mixture to exist at equilibrium, the rates of the forward and backward (reverse) reactions must be equal. In the following chemical equation, arrows point both ways to indicate equilibrium. A and B are reactant chemical species, S and T a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flux (metallurgy)
In metallurgy, a flux () is a chemical cleaning agent, flowing agent, or purifying agent. Fluxes may have more than one function at a time. They are used in both extractive metallurgy and metal joining. Some of the earliest known fluxes were sodium carbonate, potash, charcoal, coke, borax, lime, lead sulfide and certain minerals containing phosphorus. Iron ore was also used as a flux in the smelting of copper. These agents served various functions, the simplest being a reducing agent, which prevented oxides from forming on the surface of the molten metal, while others absorbed impurities into the slag, which could be scraped off the molten metal. Fluxes are also used in foundries for removing impurities from molten nonferrous metals such as aluminium, or for adding desirable trace elements such as titanium. As cleaning agents, fluxes facilitate soldering, brazing, and welding by removing oxidation from the metals to be joined. In some applications molten flux ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some sta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




.jpg)