|
Pramocaine Synthesis
Pramocaine (International Nonproprietary Name, INN and British Approved Name, BAN, also known as pramoxine or pramoxine HCI) is a topical anesthetic discovered at Abbott Laboratories in 1953 and used as an antipruritic. During research and development, pramocaine hydrochloride stood out among a series of alkoxy aryl alkamine ethers as an especially good topical local anesthetic agent. Pharmacologic study revealed it to be potent and of low acute and subacute toxicity, well tolerated by most mucous membranes and of a low sensitizing index in humans. Like other local anesthetics, pramocaine decreases the permeability of neuronal membranes to sodium ions, blocking both initiation and conduction of nerve impulses. Depolarization and repolarization of excitable neural membranes is thus inhibited, leading to numbness. Use Topical anesthetics are used to relieve pain and itching caused by conditions such as sunburn or other minor burns, insect bites or stings, poison ivy, poison oak, p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloride
In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base (e.g. an amine). An alternative name is chlorhydrate, which comes from French. An archaic alternative name is muriate, derived from hydrochloric acid's ancient name: muriatic acid. Uses Converting amines into their hydrochlorides is a common way to improve their water solubility, which can be desirable for substances used in medications. The European Pharmacopoeia lists more than 200 hydrochlorides as active ingredients in medications. These hydrochlorides, compared to free bases, may more readily dissolve in the gastrointestinal tract and be absorbed into the bloodstream more quickly. Additionally, many hydrochlorides of amines have a longer shelf-life than their respective free bases. Amine hydrochlorides represent latent forms of a more reactive free base. In this regard, formation of an amine hydrochloride confers protection. This eff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Local Anesthetics
A local anesthetic (LA) is a medication that causes absence of pain sensation. In the context of surgery, a local anesthetic creates an absence of pain in a specific location of the body without a loss of consciousness, as opposed to a general anesthetic. When it is used on specific nerve pathways (local anesthetic nerve block), paralysis (loss of muscle power) also can be achieved. Examples Short Duration & Low Potency Procaine Chloroprocaine Medium Duration & Potency Lidocaine Prilocaine High Duration & Potency Tetracaine Bupivacaine Cinchocaine Ropivacaine Clinical LAs belong to one of two classes: aminoamide and aminoester local anesthetics. Synthetic LAs are structurally related to cocaine. They differ from cocaine mainly in that they have a very low abuse potential and do not produce hypertension or (with few exceptions) vasoconstriction. They are used in various techniques of local anesthesia such as: * Topical anesthesia (surface) * Topical administration ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Local Anesthesia
Local anesthesia is any technique to induce the absence of sensation in a specific part of the body, generally for the aim of inducing local analgesia, that is, local insensitivity to pain, although other local senses may be affected as well. It allows patients to undergo surgical and dental procedures with reduced pain and distress. In many situations, such as cesarean section, it is safer and therefore superior to general anesthesia. The following terms are often used interchangeably: * ''Local anesthesia'', in a strict sense, is anesthesia of a small part of the body such as a tooth or an area of skin. * ''Regional anesthesia'' is aimed at anesthetizing a larger part of the body such as a leg or arm. * ''Conduction anesthesia'' encompasses a great variety of local and regional anesthetic techniques. Medical A local anesthetic is a drug that causes reversible local anesthesia and a loss of nociception. When it is used on specific nerve pathways (nerve block), effects such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-bromobutane
1-Bromobutane is the organobromine compound with the formula CH3(CH2)3Br. It is a colorless liquid, although impure samples appear yellowish. It is insoluble in water, but soluble in organic solvents. It is a primarily used as a source of the butyl group in organic synthesis. It is one of several isomers of butyl bromide. Synthesis Most 1-bromoalkanes are prepared by free-radical addition of hydrogen bromide to the 1-alkene. These conditions lead to the anti-Markovnikov addition, i.e. give the 1-bromo derivatives. 1-Bromobutane can also be prepared from butanol by treatment with hydrobromic acid: :CH3(CH2)3OH + HBr → CH3(CH2)3Br + H2O Reactions As a primary haloalkane, it is prone to SN2 type reactions. It is commonly used as an alkylating agent. When combined with magnesium metal in dry ether, it gives the corresponding Grignard reagent A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroquinone
Hydroquinone, also known as benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of phenol, a derivative of benzene, having the chemical formula C6H4(OH)2. It has two hydroxyl groups bonded to a benzene ring in a ''para'' position. It is a white granular solid. Substituted derivatives of this parent compound are also referred to as hydroquinones. The name "hydroquinone" was coined by Friedrich Wöhler in 1843. Production Hydroquinone is produced industrially in two main ways.Phillip M. Hudnall "Hydroquinone" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. 2005 Wiley-VCH, Weinheim. . * The most widely used route is similar to the cumene process in reaction mechanism and involves the dialkylation of benzene with propene to give 1,4-diisopropylbenzene. This compound reacts with air to afford the bis(hydroperoxide), which is structurally similar to cumene hydroperoxide and rearranges in acid to give acetone and hydroquinone. * A se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pramocaine Synthesis
Pramocaine (International Nonproprietary Name, INN and British Approved Name, BAN, also known as pramoxine or pramoxine HCI) is a topical anesthetic discovered at Abbott Laboratories in 1953 and used as an antipruritic. During research and development, pramocaine hydrochloride stood out among a series of alkoxy aryl alkamine ethers as an especially good topical local anesthetic agent. Pharmacologic study revealed it to be potent and of low acute and subacute toxicity, well tolerated by most mucous membranes and of a low sensitizing index in humans. Like other local anesthetics, pramocaine decreases the permeability of neuronal membranes to sodium ions, blocking both initiation and conduction of nerve impulses. Depolarization and repolarization of excitable neural membranes is thus inhibited, leading to numbness. Use Topical anesthetics are used to relieve pain and itching caused by conditions such as sunburn or other minor burns, insect bites or stings, poison ivy, poison oak, p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemorrhoid
Hemorrhoids (or haemorrhoids), also known as piles, are vascular structures in the anal canal. In their normal state, they are cushions that help with stool control. They become a disease when swollen or inflamed; the unqualified term ''hemorrhoid'' is often used to refer to the disease. The signs and symptoms of hemorrhoids depend on the type present. Internal hemorrhoids often result in painless, bright red rectal bleeding when defecating. External hemorrhoids often result in pain and swelling in the area of the anus. If bleeding occurs, it is usually darker. Symptoms frequently get better after a few days. A skin tag may remain after the healing of an external hemorrhoid. While the exact cause of hemorrhoids remains unknown, a number of factors that increase pressure in the abdomen are believed to be involved. This may include constipation, diarrhea, and sitting on the toilet for long periods. Hemorrhoids are also more common during pregnancy. Diagnosis is made by lookin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
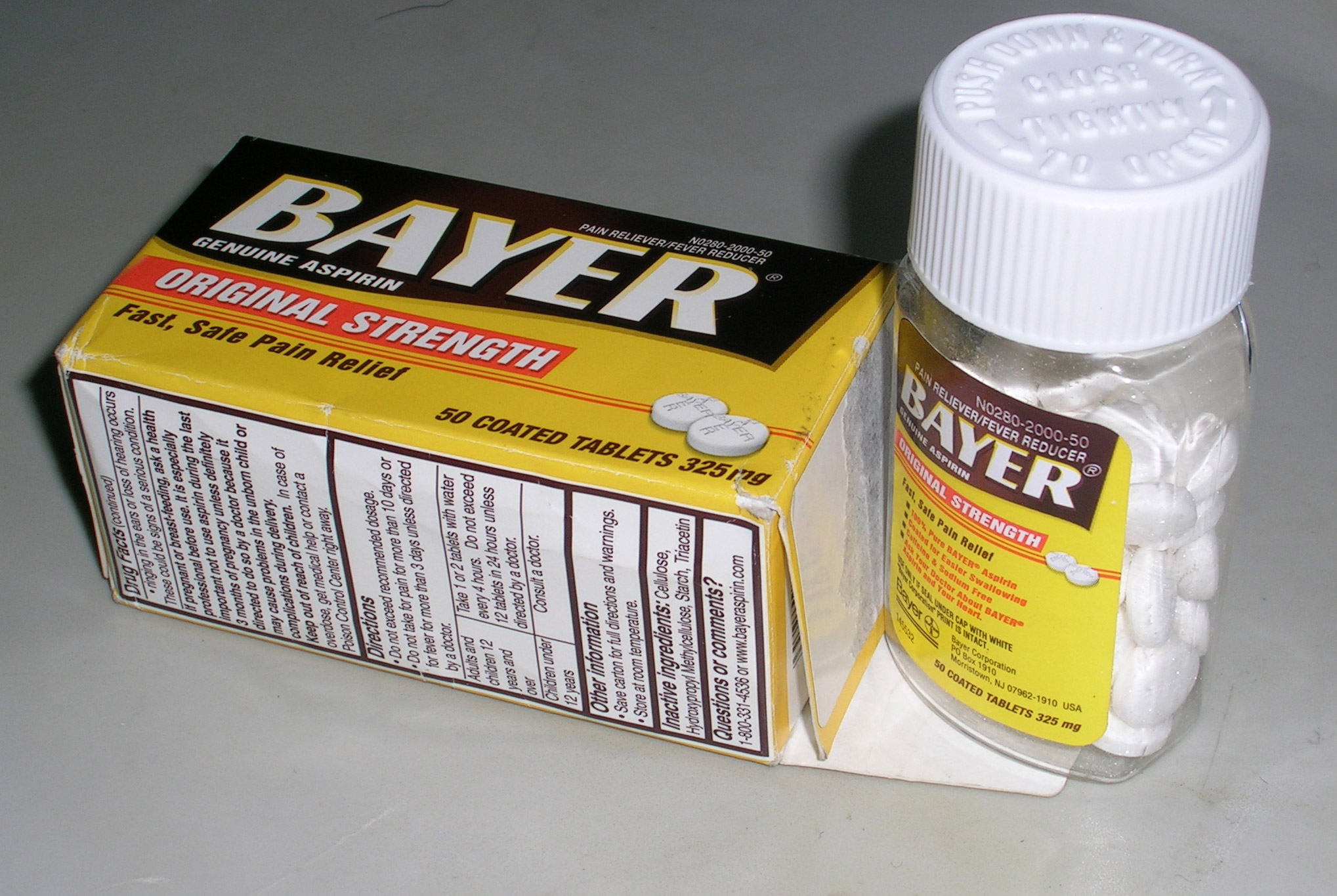
Over-the-counter Drug
Over-the-counter (OTC) drugs are medicines sold directly to a consumer without a requirement for a prescription from a healthcare professional, as opposed to prescription drugs A prescription drug (also prescription medication or prescription medicine) is a pharmaceutical drug that legally requires a medical prescription to be dispensed. In contrast, over-the-counter drugs can be obtained without a prescription. The rea ..., which may be supplied only to consumers possessing a valid prescription. In many countries, OTC drugs are selected by a regulatory agency to ensure that they contain ingredients that are safe and effective when used without a physician's care. OTC drugs are usually regulated according to their active pharmaceutical ingredient (API) rather than final products. By regulating APIs instead of specific drug formulations, governments allow manufacturers the freedom to formulate ingredients, or combinations of ingredients, into proprietary mixtures. The term ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt (chemistry)
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions. The component ions in a salt compound can be either inorganic, such as chloride (Cl−), or organic, such as acetate (). Each ion can be either monatomic, such as fluoride (F−), or polyatomic, such as sulfate (). Types of salt Salts can be classified in a variety of ways. Salts that produce hydroxide ions when dissolved in water are called ''alkali salts'' and salts that produce hydrogen ions when dissolved in water are called ''acid salts''. ''Neutral salts'' are those salts that are neither acidic nor basic. Zwitterions contain an anionic and a cationic centre in the same molecule, but are not considered salts. Examples of zwitterions are amino acids, many metabolites, peptid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calamine Lotion
Calamine, also known as calamine lotion, is a medication used to treat mild itchiness. This includes from sunburn, insect bites, poison ivy, poison oak, and other mild skin conditions. It may also help dry out skin irritation. It is applied on the skin as a cream or lotion. Side effects may include skin irritation. It is considered to be safe in pregnancy. Calamine is a combination of zinc oxide and 0.5% ferric oxide (Fe2O3). The lotion is produced with additional ingredients such as phenol and calcium hydroxide. The use of calamine lotion dates back as far as 1500 BC. It is on the World Health Organization's List of Essential Medicines. Calamine is available over-the-counter as a generic medication. Medical uses Calamine is used to treat itchiness. This includes sunburn, insect bite, or other mild skin conditions. Effectiveness The FDA recommends applying some topical over-the-counter skin products, such as calamine, to absorb the weeping of the skin caused by poi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |