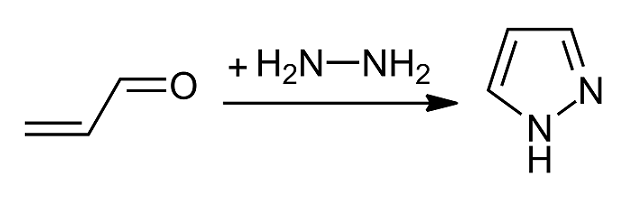|
Potassium Trispyrazolylborate
Potassium trispyrazolylborate, commonly abbreviated KTp, is the potassium salt with the formula :. This salt is the source of the trispyrazolylborate ligand. KTp is a white crystalline solid that is soluble in polar solvents, alcohols, and water. The synthesis of KTp involves potassium borohydride and pyrazole without a solvent. : The tris(pyrazolyl)borate forms octahedral coordination compounds with the formula M psub>2 with first row transition metals. KTp also forms 1:1 complexes, for example it can be converted to K pMo(CO)3 : When K pMo(CO)3is treated with butyl nitrite Butyl nitrite is the organic compound with the formula CH3(CH2)3ONO. It is an alkyl nitrite made from ''n''-butanol. Butyl nitrite is used recreationally as poppers. Synonyms include ''1-butyl nitrite'', n''-butyl nitrite'' and ''nitrous acid b ... it yields the neutral orange complex TpMo(CO)2NO. :K pMo(CO)3 BuONO→TpMo(CO)2NO+CO+KOBu References {{boron compounds Potassium compounds Boron com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trispyrazolylborate
In inorganic chemistry, the trispyrazolylborate ligand, abbreviated Tp−, is an anionic tridentate and tripodal ligand. Trispyrazolylborate refers specifically to the anion B(C3N2H3)3sup>−, but the term trispyrazolylborate refers to derivatives substituted at on the pyrazolyl rings. This family of compounds are sometimes called scorpionate ligands. Tp ligands As suggested by the resonance structures, the nitrogen centers that are not bonded to boron are basic. These centers bind to three adjacent sites of a metal such that the simple adducts have C3v symmetry. The facial bonding mode is reminiscent of cyclopentadienyl ligands, although the ligand field stabilization energy of Tp− is weaker as indicated by the fact that Fe(Tp)2 is a spin-crossover complex whereas ferrocene is low-spin. The Tp ligands are usually prepared from the reaction of pyrazole with potassium borohydride: :KBH4 + 3 C3H3N2H → K B(C3N2H3)3 + 3H2 Intermediates include the monopyrazolylborate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polar Solvents
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents ( citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical synthe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food, energy or organic micronutrients. Its chemical formula, H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. "Water" is also the name of the liquid state of H2O at standard temperature and pressure. A number of natural states of water exist. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of water is steam or water vapor. Water co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Borohydride
Borohydride refers to the anion , which is also called tetrahydroborate, and its salts. Borohydride or hydroborate is also the term used for compounds containing , where ''n'' is an integer from 0 to 3, for example cyanoborohydride or cyanotrihydroborate and triethylborohydride or triethylhydroborate . Borohydrides find wide use as reducing agents in organic synthesis. The most important borohydrides are lithium borohydride and sodium borohydride, but other salts are well known (see Table). Tetrahydroborates are also of academic and industrial interest in inorganic chemistry. History Alkali metal borohydrides were first described in 1940 by Hermann Irving Schlesinger and Herbert C. Brown. They synthesized lithium borohydride from diborane : :, where M = Li, Na, K, Rb, Cs, etc. Current methods involve reduction of trimethyl borate with sodium hydride. Structure In the borohydride anion and most of its modifications, boron has a tetrahedral structure. The reactivity of the B− ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrazole
Pyrazole is an organic compound with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ... atoms and two adjacent nitrogen atoms, which are in Arene substitution pattern, ortho-substitution. Pyrazole is a weak base, with p''K''b 11.5 (p''K''a of the conjugate acid 2.49 at 25 °C). Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol. Preparation and reactions Pyrazoles are synthesized by the reaction of α,β-unsaturated aldehydes with hydrazine and subsequent dehydrogenation: : Substituted pyrazoles are prepared by condensation of 1,3-diketones ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butyl Nitrite
Butyl nitrite is the organic compound with the formula CH3(CH2)3ONO. It is an alkyl nitrite made from n-Butanol, ''n''-butanol. Butyl nitrite is used recreationally as poppers. Synonyms include ''1-butyl nitrite'', n''-butyl nitrite'' and ''nitrous acid butyl ester''. It can be prepared by treating nitrous acid (generated ''in situ'' with n-butanol, ''n''-butanol. Applications Butyl nitrite is one of the compounds used as poppers, inhalant drugs that induce brief euphoria. It was developed by Clifford Hassing, a graduate student in Los Angeles, as a faster-acting analog of alkyl nitrite. Among the inhalants' trade names are Rush, Locker Room, and Bolt. They are sometimes marketed as "Cleaner", liquid incense, or room odorizer. It is used for its euphoric effect and for relaxing the smooth muscle, smooth muscles during sexual intercourse. See also *tert-butyl nitrite References Antianginals Antidotes Alkyl nitrites Butyl compounds {{psychoactive-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
World Scientific Publishing Company
World Scientific Publishing is an academic publisher of scientific, technical, and medical books and journals headquartered in Singapore. The company was founded in 1981. It publishes about 600 books annually, along with 135 journals in various fields. In 1995, World Scientific co-founded the London-based Imperial College Press together with the Imperial College of Science, Technology and Medicine. Company structure The company head office is in Singapore. The Chairman and Editor-in-Chief is Dr Phua Kok Khoo, while the Managing Director is Doreen Liu. The company was co-founded by them in 1981. Imperial College Press In 1995 the company co-founded Imperial College Press, specializing in engineering, medicine and information technology, with Imperial College London. In 2006, World Scientific assumed full ownership of Imperial College Press, under a license granted by the university. Finally, in August 2016, ICP was fully incorporated into World Scientific under the new imprint ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Compounds
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Compounds
Boron compounds are compounds containing the element boron. In the most familiar compounds, boron has the formal oxidation state +3. These include oxides, sulfides, nitrides, and halides. Halides The trihalides adopt a planar trigonal structure. These compounds are Lewis acids in that they readily form adducts with electron-pair donors, which are called Lewis bases. For example, fluoride (F−) and boron trifluoride (BF3) combined to give the tetrafluoroborate anion, BF4−. Boron trifluoride is used in the petrochemical industry as a catalyst. The halides react with water to form boric acid. Boron is found in nature on Earth almost entirely as various oxides of B(III), often associated with other elements. More than one hundred borate minerals contain boron in oxidation state +3. These minerals resemble silicates in some respect, although boron is often found not only in a tetrahedral coordination with oxygen, but also in a trigonal planar configuration. Unlike silicates, b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

4-3D-balls.png)