|
Porous Polymer
Porous polymers are a class of porous media materials in which monomers form 2D polymer, 2D and 3D polymers containing angstrom- to nanometer-scale pores formed by the arrangement of the monomers. They may be either crystalline or amorphous. Subclasses include covalent organic frameworks (COFs), hydrogen-bonded organic frameworks (HOFs), metal-organic frameworks (MOFs), and porous organic polymers (POPs). The subfield of chemistry specializing in porous polymers is called reticular chemistry. Covalent organic frameworks Covalent organic frameworks are crystalline porous polymers assembled from organic monomers linked through covalent bonds. Hydrogen-bonded organic frameworks Hydrogen-bonded organic frameworks are crystalline porous polymers assembled from organic monomers linked through hydrogen bonds. Metal-organic frameworks Metal-organic frameworks are crystalline porous polymers assembled from organic monomers connected by Coordination chemistry, coordination to metal atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porous Media
A porous medium or a porous material is a material containing pores (voids). The skeletal portion of the material is often called the "matrix" or "frame". The pores are typically filled with a fluid (liquid or gas). The skeletal material is usually a solid, but structures like foams are often also usefully analyzed using concept of porous media. A porous medium is most often characterised by its porosity. Other properties of the medium (e.g. permeability, tensile strength, electrical conductivity, tortuosity) can sometimes be derived from the respective properties of its constituents (solid matrix and fluid) and the media porosity and pores structure, but such a derivation is usually complex. Even the concept of porosity is only straightforward for a poroelastic medium. Often both the solid matrix and the pore network (also known as the pore space) are continuous, so as to form two interpenetrating continua such as in a sponge. However, there is also a concept of closed porosit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization. Classification Monomers can be classified in many ways. They can be subdivided into two broad classes, depending on the kind of the polymer that they form. Monomers that participate in condensation polymerization have a different stoichiometry than monomers that participate in addition polymerization: : Other classifications include: *natural vs synthetic monomers, e.g. glycine vs caprolactam, respectively *polar vs nonpolar monomers, e.g. vinyl acetate vs ethylene, respectively *cyclic vs linear, e.g. ethylene oxide vs ethylene glycol, respectively The polymerization of one kind of monomer gives a homopolymer. Many polymers are copolymers, meaning that they are derived from two different monomers. In the case of condensation polymerizations, the r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2D Polymer
A two-dimensional polymer (2DP) is a sheet-like monomolecular macromolecule consisting of laterally connected repeat units with end groups along all edges. This recent definition of 2DP is based on Hermann Staudinger's polymer concept from the 1920s. According to this, covalent long chain molecules ("Makromoleküle") do exist and are composed of a sequence of linearly connected repeat units and end groups at both termini. Moving from one dimension to two offers access to surface morphologies such as increased surface area, porous membranes, and possibly in-plane pi orbital-conjugation for enhanced electronic properties. They are distinct from other families of polymers because 2D polymers can be isolated as multilayer crystals or as individual sheets. The term 2D polymer has also been used more broadly to include linear polymerizations performed at interfaces, layered non-covalent assemblies, or to irregularly cross-linked polymers confined to surfaces or layered films. 2D polyme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3D Polymers
3-D, 3D, or 3d may refer to: Science, technology, and mathematics Relating to three-dimensionality * Three-dimensional space ** 3D computer graphics, computer graphics that use a three-dimensional representation of geometric data ** 3D film, a motion picture that gives the illusion of three-dimensional perception ** 3D modeling, developing a representation of any three-dimensional surface or object ** 3D printing, making a three-dimensional solid object of a shape from a digital model ** 3D display, a type of information display that conveys depth to the viewer ** 3D television, television that conveys depth perception to the viewer ** Stereoscopy, any technique capable of recording three-dimensional visual information or creating the illusion of depth in an image Other uses in science and technology or commercial products * 3D projection * 3D rendering * 3D scanning, making a digital representation of three-dimensional objects * 3D video game (other) * 3-D Secure, a se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Covalent Organic Framework
Covalent organic frameworks (COFs) are a class of materials that form two- or three-dimensional structures through reactions between organic precursors resulting in strong, covalent bonds to afford porous, stable, and crystalline materials. COFs emerged as a field from the overarching domain of organic materials as researchers optimized both synthetic control and precursor selection. These improvements to coordination chemistry enabled non-porous and amorphous organic materials such as organic polymers to advance into the construction of porous, crystalline materials with rigid structures that granted exceptional material stability in a wide range of solvents and conditions. Through the development of reticular chemistry, precise synthetic control was achieved and resulted in ordered, nano-porous structures with highly preferential structural orientation and properties which could be synergistically enhanced and amplified. With judicious selection of COF secondary building units (SBU ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
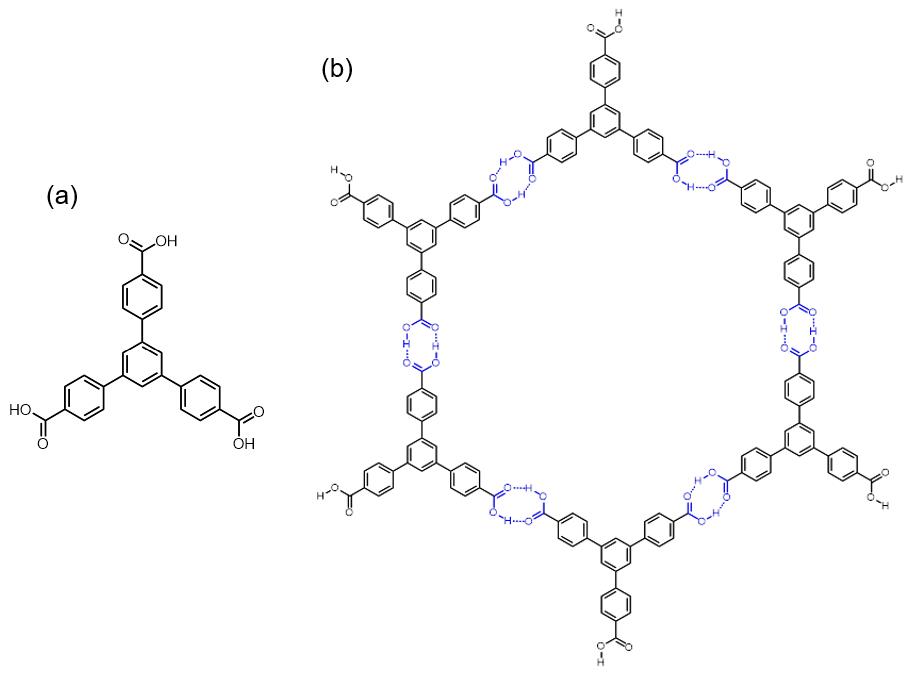
Hydrogen-bonded Organic Framework
Hydrogen-bonded organic frameworks (HOFs) are a class of porous polymers formed by hydrogen bonds among molecular monomer units to afford porosity and structural flexibility. There are diverse hydrogen bonding pair choices that could be used in HOFs construction, including identical or nonidentical hydrogen bonding donors and acceptors. For organic groups acting as hydrogen bonding units, species like carboxylic acid, amide, 2,4-diaminotriazine, and imidazole, etc., are commonly used for the formation of hydrogen bonding interaction. Compared with other organic frameworks, like Covalent organic framework, COF and Metal–organic framework, MOF, the binding force of HOFs is relatively weaker, and the activation of HOFs is more difficult than other frameworks, while the reversibility of hydrogen bonds guarantees a high crystallinity of the materials. Though the stability and pore size expansion of HOFs has potential problems, HOFs still show strong potential for applications in differen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porous Organic Polymer
Porosity or void fraction is a measure of the void (i.e. "empty") spaces in a material, and is a fraction of the volume of voids over the total volume, between 0 and 1, or as a percentage between 0% and 100%. Strictly speaking, some tests measure the "accessible void", the total amount of void space accessible from the surface (cf. closed-cell foam). There are many ways to test porosity in a substance or part, such as industrial CT scanning. The term porosity is used in multiple fields including pharmaceutics, ceramics, metallurgy, materials, manufacturing, petrophysics, hydrology, earth sciences, soil mechanics, and engineering. Void fraction in two-phase flow In gas-liquid two-phase flow, the void fraction is defined as the fraction of the flow-channel volume that is occupied by the gas phase or, alternatively, as the fraction of the cross-sectional area of the channel that is occupied by the gas phase. Void fraction usually varies from location to location in the flow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reticular Chemistry
Omar M. Yaghi ( ar, عمر مونّس ياغي; born February 9, 1965) is the James and Neeltje Tretter Chair Professor of Chemistry at the University of California, Berkeley, the Founding Director of the Berkeley Global Science Institute, and an elected member of the US National Academy of Sciences. Early life and education Yaghi was born in Amman, Jordan in 1965 to a refugee family, originally from Mandatory Palestine. He grew up in a household with many children, but only had limited access to clean water and without electricity. At the age of 15, he moved to the United States at the encouragement of his father. Although he knew little English, he began classes at Hudson Valley Community College, and later transferred to the University at Albany, SUNY to finish his college degree. He began his graduate studies at University of Illinois, Urbana-Champaign and received his PhD in 1990 under the guidance of Walter G. Klemperer. He was a National Science Foundation Postdoctora ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Covalent Bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding. Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds and three-center four-electron bonds. The term ''covalent bond'' dates from 1939. The prefix ''co-'' means ''jointly, associated in action, partnered to a lesser degree, '' etc.; thus a "co-valent bond", in essence, means that the atoms share " valence", such a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Science (journal)
''Science'', also widely referred to as ''Science Magazine'', is the peer-reviewed academic journal of the American Association for the Advancement of Science (AAAS) and one of the world's top academic journals. It was first published in 1880, is currently circulated weekly and has a subscriber base of around 130,000. Because institutional subscriptions and online access serve a larger audience, its estimated readership is over 400,000 people. ''Science'' is based in Washington, D.C., United States, with a second office in Cambridge, UK. Contents The major focus of the journal is publishing important original scientific research and research reviews, but ''Science'' also publishes science-related news, opinions on science policy and other matters of interest to scientists and others who are concerned with the wide implications of science and technology. Unlike most scientific journals, which focus on a specific field, ''Science'' and its rival ''Nature (journal), Nature'' c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted , where the solid line denotes a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are the second-row elements nitrogen (N), oxygen (O), and fluorine (F). Hydrogen bonds can be intermolecular (occurring between separate molecules) or intramolecular (occurring among parts of the same molecule). The energy of a hydrogen bond depends on the geometry, the environment, and the nature of the specific donor and acceptor atoms and can vary between 1 and 40 kcal/mol. This makes them somewhat stronger than a van der Waals interaction, and weaker than fully covalent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |