|
Poison Prevention Packaging Act Of 1970
The Poison Prevention Packaging Act of 1970 (PPPA);Pub. L. 91-601 84 Stat. 1670-74) was signed into law by U.S. President Richard Nixon on December 30, 1970. It was enacted by the 91st United States Congress. This law required the use of child-resistant packaging for prescription drugs, over-the-counter (OTC) drugs, household chemicals, and other hazardous materials that could be considered dangerous for children. Background Before the PPPA was enacted, unintentional poisonings by both medicines and common household products were considered by most pediatricians to be the leading cause of injury to children aged 5 and under. At that time there were about 500 deaths per year being reported for children aged 5 and under due to accessibility of these chemicals. The purpose of the PPPA was to protect children from ingesting harmful chemicals and prescription medications by accident. After the PPPA was implemented, deaths in children aged 5 and under went down by 1.4 per million. Thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Commerce And Trade
Commerce is the large-scale organized system of activities, functions, procedures and institutions directly and indirectly related to the exchange (buying and selling) of goods and services among two or more parties within local, regional, national or international economies. More specifically, commerce is not business, but rather the part of business which facilitates the movement and distribution of finished or unfinished but valuable goods and services from the producers to the end consumers on a large scale, as opposed to the sourcing of raw materials and manufacturing of those goods. Commerce is subtly different from trade as well, which is the final transaction, exchange or transfer of finished goods and services between a seller and an end consumer. Commerce not only includes trade as defined above, but also a series of transactions that happen between the producer and the seller with the help of the auxiliary services and means which facilitate such trade. These auxiliary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
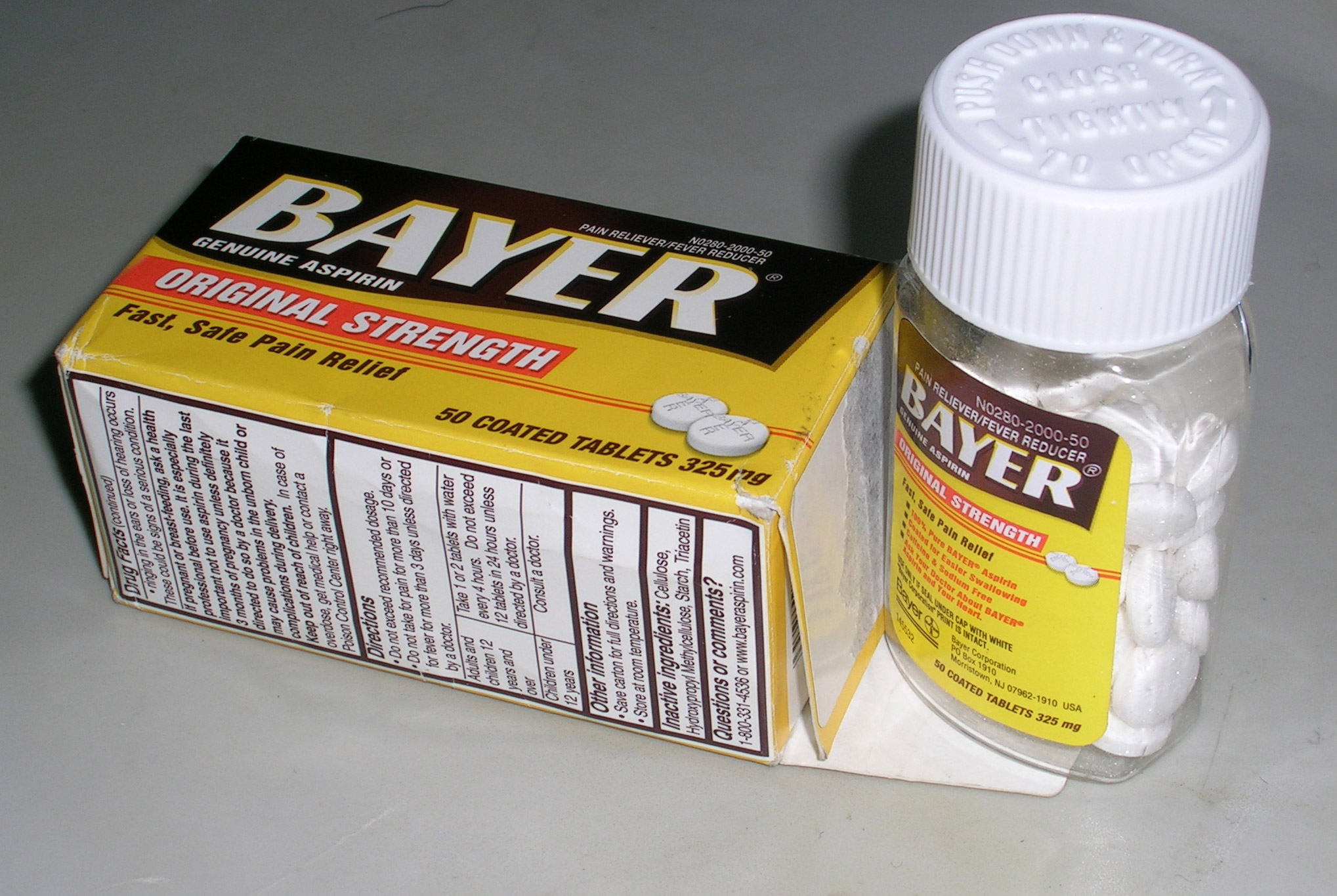
Child-resistant Packaging
Child-resistant packaging or CR packaging is special packaging used to reduce the risk of children ingesting hazardous materials. This is often accomplished by the use of a special safety cap. It is required by regulation for prescription drugs, over-the-counter medications, Nicotine Containing Electronic Cigarette devices or Refill containers that can contain Nicotine EUTPD 36.7 pesticides, and household chemicals. In some jurisdictions, ''unit packaging'' such as blister packs is also regulated for child safety. The U.S. Consumer Product Safety Commission has stated in a press release that "There is no such thing as child-proof packaging. So you shouldn't think of packaging as your primary line of defense. Rather, you should think of packaging, even child-resistant packaging, as your last line of defense." Background The child-resistant locking closure for containers was invented in 1967 by Dr. Henri Breault. A history of accidents involving children opening household packagi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Patient Safety
Patient safety is a discipline that emphasizes safety in health care through the prevention, reduction, reporting and analysis of error and other types of unnecessary harm that often lead to adverse patient events. The frequency and magnitude of avoidable adverse events, often known as patient safety incidents, experienced by patients was not well known until the 1990s, when multiple countries reported significant numbers of patients harmed and killed by medical errors. Recognizing that healthcare errors impact 1 in every 10 patients around the world, the World Health Organization (WHO) calls patient safety an endemic concern. Indeed, patient safety has emerged as a distinct healthcare discipline supported by an immature yet developing scientific framework. There is a significant transdisciplinary body of theoretical and research literature that informs the science of patient safety with mobile health apps being a growing area of research. Prevalence of adverse events Millenn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Safety
Term Given By Tushar Sharma (UPES Batch 2025) Pharmacovigilance (PV, or PhV), also known as drug safety, is the pharmaceutical science relating to the "collection, detection, assessment, monitoring, and prevention" of adverse effects with pharmaceutical products. The etymological roots for the word "pharmacovigilance" are: (Greek for drug) and (Latin for to keep watch). As such, pharmacovigilance heavily focuses on adverse drug reactions (ADR), which are defined as any response to a drug which is noxious and unintended, including lack of efficacy (the condition that this definition only applies with the doses normally used for the prophylaxis, diagnosis or therapy of disease, or for the modification of physiological disorder function was excluded with the latest amendment of the applicable legislation). Medication errors such as overdose, and misuse and abuse of a drug as well as drug exposure during pregnancy and breastfeeding, are also of interest, even without an adverse ev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Comprehensive Drug Abuse Prevention And Control Act Of 1970
The Comprehensive Drug Abuse Prevention and Control Act of 1970, , is a United States federal law that, with subsequent modifications, requires the pharmaceutical industry to maintain physical security and strict record keeping for certain types of drugs. Controlled substances are divided into five schedules (or classes) on the basis of their potential for abuse, accepted medical use, and accepted safety under medical supervision. Substances in Schedule I have a high potential for abuse, no accredited medical use, and a lack of accepted safety. From Schedules II to V, substances decrease in potential for abuse. The schedule a substance is placed in determines how it must be controlled. Prescriptions for drugs in all schedules must bear the physician's federal Drug Enforcement Administration (DEA) license number, but some drugs in Schedule V do not require a prescription. State schedules may vary from federal schedules. The Controlled Substances Act (CSA), Title II of the Compre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hazardous Substances Labeling Act
A hazard is a potential source of harm. Substances, events, or circumstances can constitute hazards when their nature would allow them, even just theoretically, to cause damage to health, life, property, or any other interest of value. The probability of that harm being realized in a specific ''incident'', combined with the magnitude of potential harm, make up its risk, a term often used synonymously in colloquial speech. Hazards can be classified in several ways; they can be classified as natural, anthropogenic, technological, or any combination, such as in the case of the natural phenomenon of wildfire becoming more common due to human-made climate change or more harmful due to changes in building practices. A common theme across many forms of hazards in the presence of stored energy that, when released, can cause damage. The stored energy can occur in many forms: chemical, mechanical, thermal hazards and by the populations that may be affected and the severity of the associated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
American Medical Association
The American Medical Association (AMA) is a professional association and lobbying group of physicians and medical students. Founded in 1847, it is headquartered in Chicago, Illinois. Membership was approximately 240,000 in 2016. The AMA's stated mission is "to promote the art and science of medicine and the betterment of public health." The Association also publishes the ''Journal of the American Medical Association'' (JAMA). The AMA also publishes a list of Physician Specialty Codes which are the standard method in the U.S. for identifying physician and practice specialties. The American Medical Association is governed by a House of Delegates as well as a board of trustees in addition to executive management. The organization maintains the AMA Code of Medical Ethics, and the AMA Physician Masterfile containing data on United States Physicians. The ''Current Procedural Terminology'' coding system was first published in 1966 and is maintained by the Association. It has also publi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C), but the agency also enforces other laws, notably Section 361 of the Public Health Service Act, as well as associated regulations. Much of this regulatory-enforcement work is not d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
91st United States Congress
The 91st United States Congress was a meeting of the legislative branch of the United States federal government, composed of the United States Senate and the United States House of Representatives. It met in Washington, DC from January 3, 1969, to January 3, 1971, during the final weeks of the presidency of Lyndon Johnson and the first two years of the first presidency of Richard Nixon. The apportionment of seats in this House of Representatives was based on the Eighteenth Census of the United States in 1960. Both chambers had a Democratic majority - albeit with losing their supermajority status in the Senate. With Richard Nixon being sworn in as President on January 20, 1969, this ended the Democrats' overall federal government trifecta that they had held since the 87th Congress. Major events *January 20, 1969: Richard M. Nixon became 37th President of the United States. Major legislation * December 30, 1969: Tax Reform Act of 1969, * December 30, 1969: Federal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drugs
Food is any substance consumed by an organism for nutritional support. Food is usually of plant, animal, or fungal origin, and contains essential nutrients, such as carbohydrates, fats, proteins, vitamins, or minerals. The substance is ingested by an organism and assimilated by the organism's cells to provide energy, maintain life, or stimulate growth. Different species of animals have different feeding behaviours that satisfy the needs of their unique metabolisms, often evolved to fill a specific ecological niche within specific geographical contexts. Omnivorous humans are highly adaptable and have adapted to obtain food in many different ecosystems. The majority of the food energy required is supplied by the industrial food industry, which produces food with intensive agriculture and distributes it through complex food processing and food distribution systems. This system of conventional agriculture relies heavily on fossil fuels, which means that the food and agricultural ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Richard Nixon
Richard Milhous Nixon (January 9, 1913April 22, 1994) was the 37th president of the United States, serving from 1969 to 1974. A member of the Republican Party, he previously served as a representative and senator from California and was the 36th vice president from 1953 to 1961 under President Dwight D. Eisenhower. His five years in the White House saw reduction of U.S. involvement in the Vietnam War, détente with the Soviet Union and China, the first manned Moon landings, and the establishment of the Environmental Protection Agency and Occupational Safety and Health Administration. Nixon's second term ended early, when he became the only president to resign from office, as a result of the Watergate scandal. Nixon was born into a poor family of Quakers in a small town in Southern California. He graduated from Duke Law School in 1937, practiced law in California, then moved with his wife Pat to Washington in 1942 to work for the federal government. After active duty ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
United States Statutes At Large
The ''United States Statutes at Large'', commonly referred to as the ''Statutes at Large'' and abbreviated Stat., are an official record of Acts of Congress and concurrent resolutions passed by the United States Congress. Each act and resolution of Congress is originally published as a slip law, which is classified as either public law (abbreviated Pub.L.) or private law (Pvt.L.), and designated and numbered accordingly. At the end of a Congressional session, the statutes enacted during that session are compiled into bound books, known as "session law" publications. The session law publication for U.S. Federal statutes is called the ''United States Statutes at Large''. In that publication, the public laws and private laws are numbered and organized in chronological order. U.S. Federal statutes are published in a three-part process, consisting of slip laws, session laws (''Statutes at Large''), and codification ('' United States Code''). Codification Large portions of public l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)