|
Platinum(IV) Chloride
Platinum(IV) chloride is the inorganic compound of platinum and chlorine with the empirical formula PtCl4. This brown solid features platinum in the 4+ oxidation state. Structure Typical of Pt(IV), the metal centers adopt an octahedral coordination geometry, . This geometry is achieved by forming a polymer wherein half of the chloride ligands bridge between the platinum centers. Because of its polymeric structure, PtCl4 dissolves only upon breaking the chloride bridging ligands. Thus, addition of HCl give H2PtCl6. Lewis base adducts of Pt(IV) of the type cis-PtCl4L2 are known, but most are prepared by oxidation of the Pt(II) derivatives. Formation and reactions PtCl4 is mainly encountered in the handling of chloroplatinic acid, obtained by dissolving of Pt metal in aqua regia. Heating H2PtCl6 gives PtCl4: :H2PtCl6 → PtCl4 + 2 HCl If excess acids are removed, PtCl4 crystallizes from aqueous solutions in large red crystals of pentahydrate PtCl4·5(H2O), which can be dehydrated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour. Acetone is miscible with water and serves as an important organic solvent in its own right, in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and production of methyl methacrylate (and from that PMMA) as well as bisphenol A.Acetone World Petrochemicals report, January 2010Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. It is a common building block in |
Octahedral Molecular Geometry
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix ''octa''. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group Oh. Examples of octahedral compounds are sulfur hexafluoride SF6 and molybdenum hexacarbonyl Mo(CO)6. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, , which is not octahedral in the mathematical sense due to the orientation of the bonds, is referred to as octahedral. The concept of octahedral coordination geometry was developed by Alfred Wern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorides
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word ''chloride'' may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Electronic properties A chloride ion (diameter 167 pm) is much larger than ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grignard Reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . They are a subclass of the organomagnesium compounds. Grignard compounds are popular reagents in organic synthesis for creating new carbon-carbon bonds. For example, when reacted with another halogenated compound in the presence of a suitable catalyst, they typically yield and the magnesium halide as a byproduct; and the latter is insoluble in the solvents normally used. In this aspect, they are similar to organolithium reagents. Pure Grignard reagents are extremely reactive solids. They are normally handled as solutions in solvents such as diethyl ether or tetrahydrofuran; which are relatively stable as long as water is excluded. In such a medium, a Grignard reagent is invariably present as a complex with the magnesium atom conn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroplatinic Acid
Chloroplatinic acid (also known as hexachloroplatinic acid) is an inorganic compound with the formula 3Osub>2 tCl6H2O)''x'' (0 ≤ ''x'' ≤ 6). A red solid, it is an important commercial source of platinum, usually as an aqueous solution. Although often written in shorthand as H2PtCl6, it is the hydronium (H3O+) salt of the hexachloroplatinate anion (). Hexachloroplatinic acid is highly hygroscopic. Production Hexachloroplatinic acid may be produced via a variety of methods. The most common of these methods involves dissolution of platinum in aqua regia. Other methods include exposing an aqueous suspension of platinum particles to chlorine gas, or via electrolysis. When produced by the aqua regia route, hexachloroplatinic acid is thought to arise by the following equation: The resulting orange/red solution can be evaporated to produce brownish red crystals. Some authors suggest that hexachloroplatinic acid produced using this method is contaminated with nitrosonium hex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystal, crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns that repeat along the principal directions of Three-dimensional space (mathematics), three-dimensional space in matter. The smallest group of particles in the material that constitutes this repeating pattern is the unit cell of the structure. The unit cell completely reflects the symmetry and structure of the entire crystal, which is built up by repetitive Translation (geometry), translation of the unit cell along its principal axes. The translation vectors define the nodes of the Bravais lattice. The lengths of the principal axes, or edges, of the unit cell and the angles between them are the lattice constants, also called ''lattice parameters'' or ''cell parameters''. The symmetry properties of the crystal are described by the con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platinum(IV)-chloride-CM-3D-balls
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver". Platinum is a member of the platinum group of elements and group 10 of the periodic table of elements. It has six naturally occurring isotopes. It is one of the rarer elements in Earth's crust, with an average abundance of approximately 5 μg/kg. It occurs in some nickel and copper ores along with some native deposits, mostly in South Africa, which accounts for ~80% of the world production. Because of its scarcity in Earth's crust, only a few hundred tonnes are produced annually, and given its important uses, it is highly valuable and is a major precious metal commodity. Platinum is one of the least reactive metals. It has remarkable resistance to corrosion, even at high temperatures, and is therefore considered a noble metal. Consequentl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adduct
An adduct (from the Latin ''adductus'', "drawn toward" alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is considered a distinct molecular species. Examples include the addition of sodium bisulfite to an aldehyde to give a sulfonate. It can just be considered as a single product resulting from the direct combination of different molecules which comprises all the reactant molecules' atoms. Adducts often form between Lewis acids and Lewis bases. A good example is the formation of adducts between the Lewis acid borane and the oxygen atom in the Lewis bases, tetrahydrofuran (THF): BH3·O(CH2)4 or diethyl ether: BH3·O(CH3CH2)2. Many Lewis acids and Lewis bases reacting in the gas phase or in non-aqueous solvents to form adducts have been examined in the ECW model. Trimethylboron, trimethyltin chloride and bis(hexaflu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Base
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane (Me3B) is a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH3 and Me3B, a lone pair from NH3 will form a dative bond with the empty orbital of Me3B to form an adduct NH3•BMe3. The terminology refers to the contributions of Gilbert N. Lewis. From p. 142: "We are inclined to think of substances as pos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
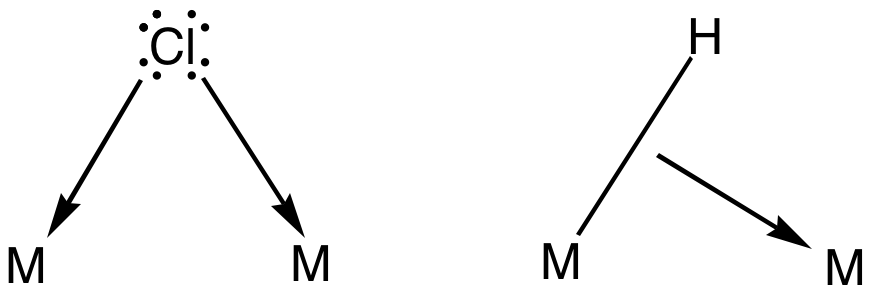
Bridging Ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals. In naming a complex wherein a single atom bridges two metals, the bridging ligand is preceded by the Greek letter mu, μ, with a subscript number denoting the number of metals bound to the bridging ligand. μ2 is often denoted simply as μ. When describing coordination complexes care should be taken not to confuse μ with η ('eta'), which relates to hapticity. Ligands that are not bridging are called terminal ligands. List of bridging ligands Virtually all ligands are known to bridge, with the exception of amines and ammonia. Common bridging ligands include most of the common anions. Many simple organic ligands form str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and hydrochloric acid (in the form of ). However ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |