|
Physical Process
Physical changes are changes affecting the form of a chemical substance, but not its chemical composition. Physical changes are used to separate mixtures into their component compounds, but can not usually be used to separate compounds into chemical elements or simpler compounds.Zumdahl, Steven S. and Zumdahl, Susan A. (2000), ''Chemistry,'' Houghton Mifflin, 5th ed., p. 27 Physical changes occur when objects or substances undergo a change that does not change their chemical composition. This contrasts with the concept of chemical change in which the composition of a substance changes or one or more substances combine or break up to form new substances. In general a physical change is reversible using physical means. For example, salt dissolved in water can be recovered by allowing the water to evaporate. A physical change involves a change in physical properties. Examples of physical properties include melting, transition to a gas, change of strength, change of durability ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Substance
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent Chemical element, elements by physical separation methods, i.e., without breaking chemical bonds. Chemical substances can be simple substances (substances consisting of a single chemical element), chemical compounds, or alloys. Chemical substances are often called 'pure' to set them apart from mixtures. A common example of a chemical substance is pure Water (molecule), water; it has the same properties and the same atomic ratio, ratio of hydrogen to oxygen whether it is isolated from a river or made in a laboratory. Other chemical substances commonly encountered in pure form are diamond (carbon), gold, Edible salt, table salt (sodium chloride) and refined sugar (sucrose). However, in practice, no substance is entirely pure, and chemical purity is specified according to the intended use of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Irreversible Process
In science, a process that is not reversible is called irreversible. This concept arises frequently in thermodynamics. All complex natural processes are irreversible, although a phase transition at the coexistence temperature (e.g. melting of ice cubes in water) is well approximated as reversible. In thermodynamics, a change in the thermodynamic state of a system and all of its surroundings cannot be precisely restored to its initial state by infinitesimal changes in some property of the system without expenditure of energy. A system that undergoes an irreversible process may still be capable of returning to its initial state. Because entropy is a state function, the change in entropy of the system is the same whether the process is reversible or irreversible. However, the impossibility occurs in restoring the environment to its own initial conditions. An irreversible process increases the total entropy of the system and its surroundings. The second law of thermodynamics can be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sandcastle
Sand art is the practice of modelling sand into an artistic form, such as sand brushing, sand sculpting, sand painting, or creating sand bottles. A sandcastle is a type of sand sculpture resembling a miniature building, often a castle. The drip castle variation uses wet sand that is dribbled down to form organic shapes before the sands dries. Most sand play takes place on sandy beaches, where the two basic building ingredients, sand and water, are available in abundance. Some sand play occurs in dry sandpits and sandboxes, though mostly by children and rarely for art forms. Tidal beaches generally have sand that limits height and structure because of the shape of the sand grains. Good sculpture sand is somewhat dirty, having silt and clay that helps lock the irregular-shaped sand grains together. Sand castles are typically made by children for fun, but there are also sand-sculpture contests for adults that involve large, complex constructions. The largest sandcastle made in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hammer
A hammer is a tool, most often a hand tool, consisting of a weighted "head" fixed to a long handle that is swung to deliver an impact to a small area of an object. This can be, for example, to drive nails into wood, to shape metal (as with a forge), or to crush rock. Hammers are used for a wide range of driving, shaping, breaking and non-destructive striking applications. Traditional disciplines include carpentry, blacksmithing, warfare, and percussive musicianship (as with a gong). Hammering is use of a hammer in its strike capacity, as opposed to prying with a secondary claw or grappling with a secondary hook. Carpentry and blacksmithing hammers are generally wielded from a stationary stance against a stationary target as gripped and propelled with one arm, in a lengthy downward planar arc—downward to add kinetic energy to the impact—pivoting mainly around the shoulder and elbow, with a small but brisk wrist rotation shortly before impact; for extreme i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buckminsterfullerene
Buckminsterfullerene is a type of fullerene with the formula C60. It has a cage-like fused-ring structure (truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a soccer ball. Each of its 60 carbon atoms is bonded to its three neighbors. Buckminsterfullerene is a black solid that dissolves in hydrocarbon solvents to produce a violet solution. The compound was discovered in 1985 and has received intense study, although few real world applications have been found. Occurrence Buckminsterfullerene is the most common naturally occurring fullerene. Small quantities of it can be found in soot. It also exists in space. Neutral C60 has been observed in planetary nebulae and several types of star. The ionised form, C60+, has been identified in the interstellar medium, where it is the cause of several absorption features known as diffuse interstellar bands in the near-infrared. History Theoretical predictions of buckyball molecules appeared i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fullerene
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ellipsoid, tube, or many other shapes and sizes. Graphene (isolated atomic layers of graphite), which is a flat mesh of regular hexagonal rings, can be seen as an extreme member of the family. Fullerenes with a closed mesh topology are informally denoted by their empirical formula C''n'', often written C''n'', where ''n'' is the number of carbon atoms. However, for some values of ''n'' there may be more than one isomer. The family is named after buckminsterfullerene (C60), the most famous member, which in turn is named after Buckminster Fuller. The closed fullerenes, especially C60, are also informally called buckyballs for their resemblance to the standard ball of association football ("soccer"). Nested closed fullerenes have been ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
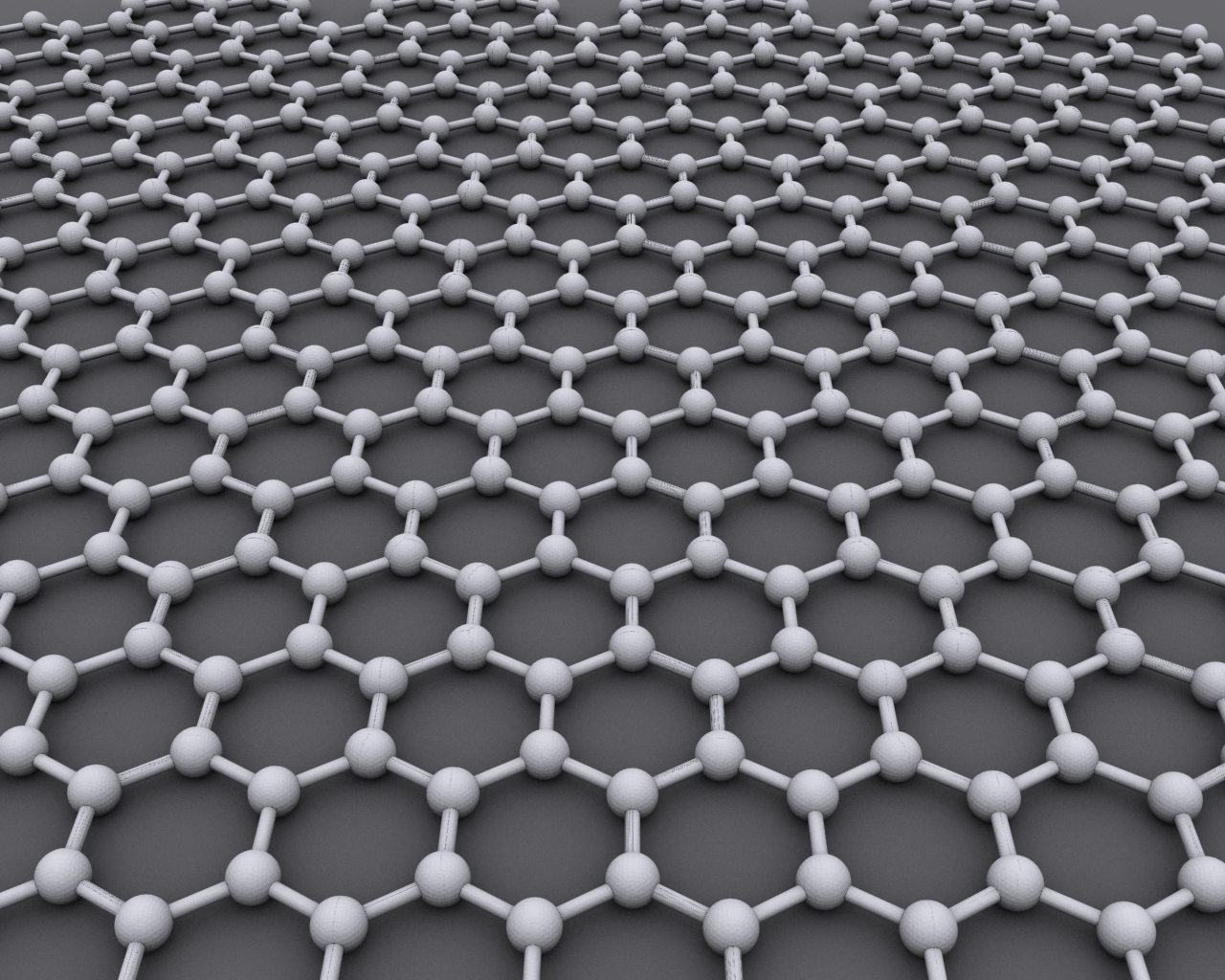
Graphene
Graphene () is an allotrope of carbon consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice nanostructure. "Carbon nanostructures for electromagnetic shielding applications", Mohammed Arif Poothanari, Sabu Thomas, et al., ''Industrial Applications of Nanomaterials'', 2019. "Carbon nanostructures include various low-dimensional allotropes of carbon including carbon black (CB), carbon fiber, carbon nanotubes (CNTs), fullerene, and graphene." The name is derived from "graphite" and the suffix -ene, reflecting the fact that the graphite allotrope of carbon contains numerous double bonds. Each atom in a graphene sheet is connected to its three nearest neighbors by a strong σ-bond, and contributes to a valence band one electron that extends over the whole sheet. This is the same type of b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
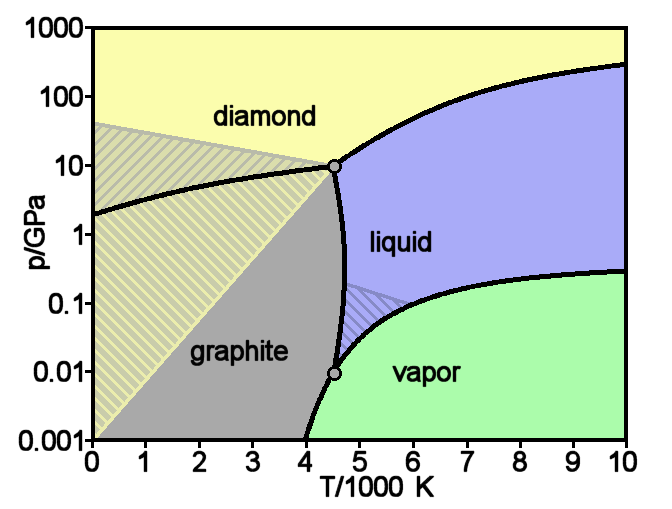
Graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large scale (300 kton/year, in 1989) for uses in pencils, lubricants, and electrodes. Under high pressures and temperatures it converts to diamond. It is a weak conductor of heat and electricity. Types and varieties Natural graphite The principal types of natural graphite, each occurring in different types of ore deposits, are * Crystalline small flakes of graphite (or flake graphite) occurs as isolated, flat, plate-like particles with hexagonal edges if unbroken. When broken the edges can be irregular or angular; * Amorphous graphite: very fine flake graphite is sometimes called amorphous; * Lump graphite (or vein graphite) occurs in fissure veins or fractures and appears as massive platy intergrowths of fibrous or acicular cry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamond
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. They are also the reason that diamond anvil cells can subject materials to pressures found deep in the Earth. Because the arrangement of atoms in diamond is extremely rigid, few types of impurity can contaminate it (two exceptions are boron and nitrogen). Small numbers of defects or impurities (about one per million of lattice atoms) color diamond blue (boron), yellow (nitrogen), brown (defects), green (radiation exposure), purple, pink, orange, or red. Diamond also has a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of Earth's crust. Three isotopes occur naturally, C and C being stable, while C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the few elements known since antiquity. Carbon is the 15th most abundant element in the Earth's crust, and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual ability to form polymers at the temperatures commonly encountered on Earth, enables this element to serve as a common element of Carbon-based life, all known life. It is the second most abundant element in the human body by mass (about 18.5%) after oxygen. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferromagnetism
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials are the familiar metals noticeably attracted to a magnet, a consequence of their large magnetic permeability. Magnetic permeability describes the induced magnetization of a material due to the presence of an ''external'' magnetic field, and it is this temporarily induced magnetization inside a steel plate, for instance, which accounts for its attraction to the permanent magnet. Whether or not that steel plate acquires a permanent magnetization itself, depends not only on the strength of the applied field, but on the so-called coercivity of that material, which varies greatly among ferromagnetic materials. In physics, several different types of material magnetism are distinguished. Ferromagnetism (along with the similar effect ferrimagnetism ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sublimation (phase Transition)
Sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state. Sublimation is an endothermic process that occurs at temperatures and pressures below a substance's triple point in its phase diagram, which corresponds to the lowest pressure at which the substance can exist as a liquid. The reverse process of sublimation is deposition or desublimation, in which a substance passes directly from a gas to a solid phase. Sublimation has also been used as a generic term to describe a solid-to-gas transition (sublimation) followed by a gas-to-solid transition ( deposition). While vaporization from liquid to gas occurs as evaporation from the surface if it occurs below the boiling point of the liquid, and as boiling with formation of bubbles in the interior of the liquid if it occurs at the boiling point, there is no such distinction for the solid-to-gas transition which always occurs as sublimation from the surface. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |