|
Photoinitiator
A photoinitiator is a molecule that creates reactive species (free radicals, cations or anions) when exposed to radiation (UV or visible). Synthetic photoinitiators are key components in photopolymers (for example, photo-curable coatings, adhesives and dental restoratives). Some small molecules in the atmosphere can also act as photoinitiators by decomposing to give free radicals (in photochemical smog). For instance, nitrogen dioxide (NO2) is produced in large quantities by gasoline-burning internal combustion engines. NO2 in the troposphere gives smog its brown coloration and catalyzes production of toxic ground-level ozone (O3). Molecular oxygen (O2) also serves as a photoinitiator in the stratosphere, breaking down into atomic oxygen and combining with O2 in order to form the ozone in the ozone layer. Reactions Photoinitators can create reactive species by different pathways including photodissociation and electron transfer. As an example of dissociation, hydrogen peroxide ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photopolymers
A photopolymer or light-activated resin is a polymer that changes its properties when exposed to light, often in the ultraviolet or visible region of the electromagnetic spectrum. These changes are often manifested structurally, for example hardening of the material occurs as a result of cross-linking when exposed to light. An example is shown below depicting a mixture of monomers, oligomers, and photoinitiators that conform into a hardened polymeric material through a process called curing. A wide variety of technologically useful applications rely on photopolymers; for example, some enamels and varnishes depend on photopolymer formulation for proper hardening upon exposure to light. In some instances, an enamel can cure in a fraction of a second when exposed to light, as opposed to thermally cured enamels which can require half an hour or longer. Curable materials are widely used for medical, printing, and photoresist technologies. Changes in structural and chemical properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
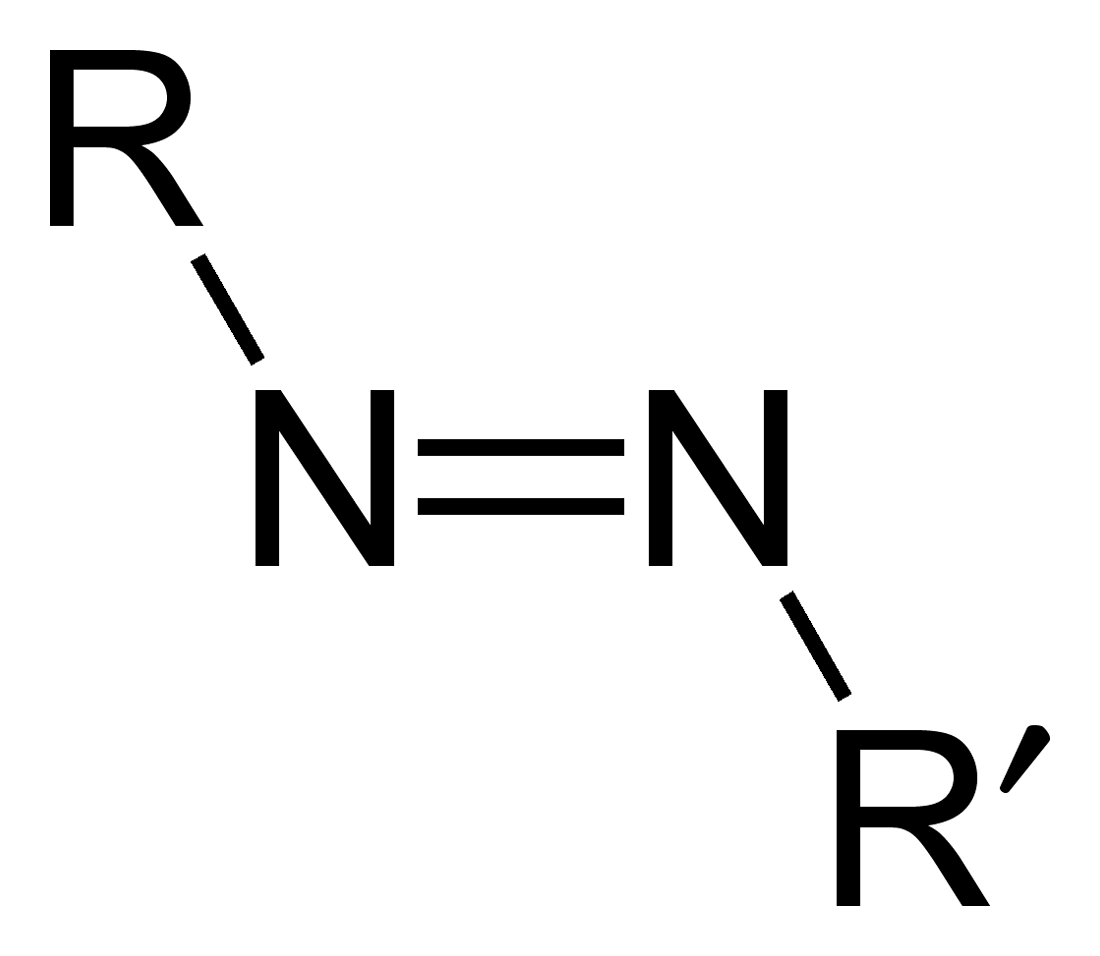
Azo Compounds
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene." The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the ''trans'' isomer, but upon illumination, converts to the ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic substitution reaction where an aryl diazonium cation is attacked by another aryl ring, especially those substituted with electron-donating groups: :ArN2+ + Ar'H -> ArN=NAr' + H+ Since diazon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical Initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions. These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical initiators are utilized in industrial processes such as polymer synthesis. Typical examples are molecules with a nitrogen-halogen bond, azo compounds, and organic and inorganic peroxides. Main types of initiation reaction *Halogens undergo homolytic fission relatively easily. Chlorine, for example, gives two chlorine radicals (Cl•) by irradiation with ultraviolet light. This process is used for chlorination of alkanes. *Azo compounds (R- N=N-R') can be the precursor of two carbon-centered radicals (R• and R'•) and nitrogen gas upon heating and/or by irradiation. For example, AIBN and ABCN yield isobutyronitrile and cyclohexanecarbonitrile radicals, respectively. : *Organic peroxides each have a peroxide bond (- O-O-), which is re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cutibacterium Acnes
''Cutibacterium acnes'' (formerly ''Propionibacterium acnes'') is the relatively slow-growing, typically aerotolerant anaerobic, gram-positive bacterium (rod) linked to the skin condition of acne; it can also cause chronic blepharitis and endophthalmitis, the latter particularly following intraocular surgery. Its genome has been sequenced and a study has shown several genes can generate enzymes for degrading skin and proteins that may be immunogenic (activating the immune system). The species is largely commensal and part of the skin flora present on most healthy adult humans' skin. It is usually just barely detectable on the skin of healthy preadolescents. It lives, among other things, primarily on fatty acids in sebum secreted by sebaceous glands in the follicles. It may also be found throughout the gastrointestinal tract. Originally identified as ''Bacillus acnes'', it was later named ''Propionibacterium acnes'' for its ability to generate propionic acid. In 2016, ''P. acne ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formation Of Radicals From AIBN
Formation may refer to: Linguistics * Back-formation, the process of creating a new lexeme by removing or affixes * Word formation, the creation of a new word by adding affixes Mathematics and science * Cave formation or speleothem, a secondary mineral deposit formed in a cave * Class formation, a topological group acting on a module satisfying certain conditions * Formation (group theory), a class of groups that is closed under some operations * Formation constant, an equilibrium constant for the formation of a complex in solution * Formation enthalpy, standard heat of formation of a compound * Formation (group theory), a class of groups * Formation (geology), a formally named rock stratum or geological unit * Formation of rocks, how rocks are formed * Formation and evolution of the Solar System, history of the Solar System * Rock formation, an isolated, scenic, or spectacular surface rock outcrop * Vegetation formation, a concept used to classify vegetation communities M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozone Layer
The ozone layer or ozone shield is a region of Earth's stratosphere that absorbs most of the Sun's ultraviolet radiation. It contains a high concentration of ozone (O3) in relation to other parts of the atmosphere, although still small in relation to other gases in the stratosphere. The ozone layer contains less than 10 parts per million of ozone, while the average ozone concentration in Earth's atmosphere as a whole is about 0.3 parts per million. The ozone layer is mainly found in the lower portion of the stratosphere, from approximately above Earth, although its thickness varies seasonally and geographically. The ozone layer was discovered in 1913 by the French physicists Charles Fabry and Henri Buisson. Measurements of the sun showed that the radiation sent out from its surface and reaching the ground on Earth is usually consistent with the spectrum of a black body with a temperature in the range of , except that there was no radiation below a wavelength of about 310&n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (•N=O or •NO). Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding. An important intermediate in industrial chemistry, nitric oxide forms in combustion systems and can be generated by lightning in thunderstorms. In mammals, including humans, nitric oxide is a signaling molecule in many physiological and pathological processes. It was proclaimed the "Molecule of the Year" in 1992. The 1998 Nobel Prize in Physiology or Medicine was awarded for discovering nitric oxide's role as a cardiovascular signalling molecule. Nitric oxide should not be confused with nitrogen dioxide (NO2), a brown gas and major air pollutant, or with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Dioxide
Nitrogen dioxide is a chemical compound with the formula . It is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year for use primarily in the production of fertilizers. At higher temperatures it is a reddish-brown gas. It can be fatal if inhaled in large quantities. Nitrogen dioxide is a paramagnetic, bent molecule with C2v molecular symmetry, point group symmetry. It is included in the NOx, NOx family of air pollution, atmospheric pollutants. Properties Nitrogen dioxide is a reddish-brown gas with a pungent, acrid odor above , becomes a yellowish-brown liquid below , and converts to the colorless dinitrogen tetroxide () below . The chemical bond, bond length between the nitrogen atom and the oxygen atom is 119.7 picometre, pm. This bond length is consistent with a bond order between one and two. Unlike ozone, O3, the ground state, ground electronic state of nitrogen dioxide is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |