|
Photochromic
Photochromism is the reversible transformation of a chemical species ( photoswitch) between two forms by the absorption of electromagnetic radiation ( photoisomerization), where the two forms have different absorption spectra. In plain language, this can be described as a reversible change of color upon exposure to light. Applications Sunglasses One of the most famous reversible photochromic applications is color changing lenses for sunglasses. The largest limitation in using photochromic technology is that the materials cannot be made stable enough to withstand thousands of hours of outdoor exposure so long-term outdoor applications are not appropriate at this time. The switching speed of photochromic dyes is highly sensitive to the rigidity of the environment around the dye. As a result, they switch most rapidly in solution and slowest in the rigid environment like a polymer lens. In 2005 it was reported that attaching flexible polymers with low glass transition temperature ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photochromic Lens
A photochromic lens is an optical lens that darkens on exposure to light of sufficiently high frequency, most commonly ultraviolet (UV) radiation. In the absence of activating light, the lenses return to their clear state. Photochromic lenses may be made of polycarbonate, or another plastic. Glass lenses use visible light to darken. They are principally used in glasses that are dark in bright sunlight, but clear, or more rarely, lightly tinted in low ambient light conditions. They darken significantly within about a minute of exposure to bright light and take somewhat longer to clear. A range of clear and dark transmittances is available. In one sort of technology, molecules of silver chloride or another silver halide are embedded in photochromic lenses. They are transparent to visible light without significant ultraviolet component, which is normal for artificial lighting. In another sort of technology, organic photochromic molecules, when exposed to ultraviolet (UV) rays as in d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoswitch
A photoswitch is a type of molecule that can change its structural geometry and chemical properties upon irradiation with electromagnetic radiation. Although often used interchangeably with the term molecular machine, a switch does not perform work upon a change in its shape whereas a machine does. However, photochromic compounds are the necessary building blocks for light driven molecular motors and machines. Upon irradiation with light, photoisomerization about double bonds in the molecule can lead to changes in the cis- or trans- configuration. These photochromic molecules are being considered for a range of applications. Chemical structures and properties A photochromic compound can change its configuration or structure upon irradiation with light. Several examples of photochromic compounds include: azobenzene, spiropyran, merocyanine, diarylethene, spirooxazine, fulgide, hydrazone, nobormadiene, thioindigo, acrylamide-azobenzene-quaternary ammonia, donor-acceptor Stenhou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3D Optical Data Storage
3D optical data storage is any form of optical data storage in which information can be recorded or read with three-dimensional resolution (as opposed to the two-dimensional resolution afforded, for example, by CD). This innovation has the potential to provide petabyte-level mass storage on DVD-sized discs (120mm). Data recording and readback are achieved by focusing lasers within the medium. However, because of the volumetric nature of the data structure, the laser light must travel through other data points before it reaches the point where reading or recording is desired. Therefore, some kind of nonlinearity is required to ensure that these other data points do not interfere with the addressing of the desired point. No commercial product based on 3Doptical data storage has yet arrived on the mass market, although several companies are actively developing the technology and claim that it may become available 'soon'. Overview Current optical data storage media, such as the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Switch
A molecular switch is a molecule that can be reversibly shifted between two or more stable states. The molecules may be shifted between the states in response to environmental stimuli, such as changes in pH, light, temperature, an electric current, microenvironment, or in the presence of ions and other ligands. In some cases, a combination of stimuli is required. The oldest forms of synthetic molecular switches are pH indicators, which display distinct colors as a function of pH. Currently synthetic molecular switches are of interest in the field of nanotechnology for application in molecular computers or responsive drug delivery systems. Molecular switches are also important in biology because many biological functions are based on it, for instance allosteric regulation and vision. They are also one of the simplest examples of molecular machines. Biological molecular switches In cellular biology, proteins act as intracellular signaling molecules by activating another protein ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sunglasses
Sunglasses or sun glasses (informally called shades or sunnies; more names Sunglasses#Other names, below) are a form of Eye protection, protective eyewear designed primarily to prevent bright sunlight and high-energy visible light from damaging or discomforting the eyes. They can sometimes also function as a visual aid, as variously termed spectacles or glasses exist, featuring lenses that are colored, polarizer, polarized or darkened. In the early 20th century, they were also known as sun cheaters (cheaters then being an United States, American slang term for glasses). Since the 1930s, sunglasses have been a popular fashion accessory, especially on the beach. The American Optometric Association recommends wearing sunglasses that block ultraviolet radiation (UV) whenever a person is in the sunlight to protect the eyes from UV and blue light, which can cause several #Protection, serious eye problems. Their usage is mandatory immediately after some surgical procedures, such as L ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supramolecular Chemistry
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects. Important concepts advanced by supramolecular chemistry include molecular self-assembly, molecular folding, molecular recognition, host–guest chemistry, mechanically-interlocked molecular architectures, and dynamic covalent chemistry. The s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photochemical Reaction
Organic photochemistry encompasses organic reactions that are induced by the action of light. The absorption of ultraviolet light by organic molecules often leads to reactions. In the earliest days, sunlight was employed, while in more modern times ultraviolet lamps are employed. Organic photochemistry has proven to be a very useful synthetic tool. Complex organic products can be obtained simply. History Early examples were often uncovered by the observation of precipitates or color changes from samples that were exposed to sunlights. The first reported case was by Ciamician that sunlight converted santonin to a yellow photoproduct: An early example of a precipitate was the photodimerization of anthracene, characterized by Yulii Fedorovich Fritzsche and confirmed by Elbs. Similar observations focused on the dimerization of cinnamic acid to truxillic acid. Many photodimers are now recognized, e.g. pyrimidine dimer, thiophosgene, diamantane. Another example was uncovered by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absorption Band
According to quantum mechanics, atoms and molecules can only hold certain defined quantities of energy, or exist in specific states. When such quanta of electromagnetic radiation are emitted or absorbed by an atom or molecule, energy of the radiation changes the state of the atom or molecule from an initial state to a final state. An absorption band is a range of wavelengths, frequencies or energies in the electromagnetic spectrum which are characteristic of a particular transition from initial to final state in a substance. Overview According to quantum mechanics, atoms and molecules can only hold certain defined quantities of energy, or exist in specific states. When electromagnetic radiation is absorbed by an atom or molecule, the energy of the radiation changes the state of the atom or molecule from an initial state to a final state. The number of states in a specific energy range is discrete for gaseous or diluted systems, with discrete energy levels. Condensed sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electromagnetic Spectrum
The electromagnetic spectrum is the range of frequencies (the spectrum) of electromagnetic radiation and their respective wavelengths and photon energies. The electromagnetic spectrum covers electromagnetic waves with frequencies ranging from below one hertz to above 1025 hertz, corresponding to wavelengths from thousands of kilometers down to a fraction of the size of an atomic nucleus. This frequency range is divided into separate bands, and the electromagnetic waves within each frequency band are called by different names; beginning at the low frequency (long wavelength) end of the spectrum these are: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays at the high-frequency (short wavelength) end. The electromagnetic waves in each of these bands have different characteristics, such as how they are produced, how they interact with matter, and their practical applications. There is no known limit for long and short wavelengths. Extre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomerization. When the isomerization occurs intramolecularly it may be called a rearrangement reaction. When the activation energy for the isomerization reaction is sufficiently small, both isomers will exist in a temperature-dependent equilibrium with each other. Many values of the standard free energy difference, \Delta G^\circ, have been calculated, with good agreement between observed and calculated data. Examples and applications Alkanes Skeletal isomerization occurs in the cracking process, used in the petrochemical industry. As well as reducing the average chain length, straight-chain hydrocarbons are converted to branched isomers in the process, as illustrated the following reaction of ''n''-butane to ''i''-butane. :\overset -> \ov ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
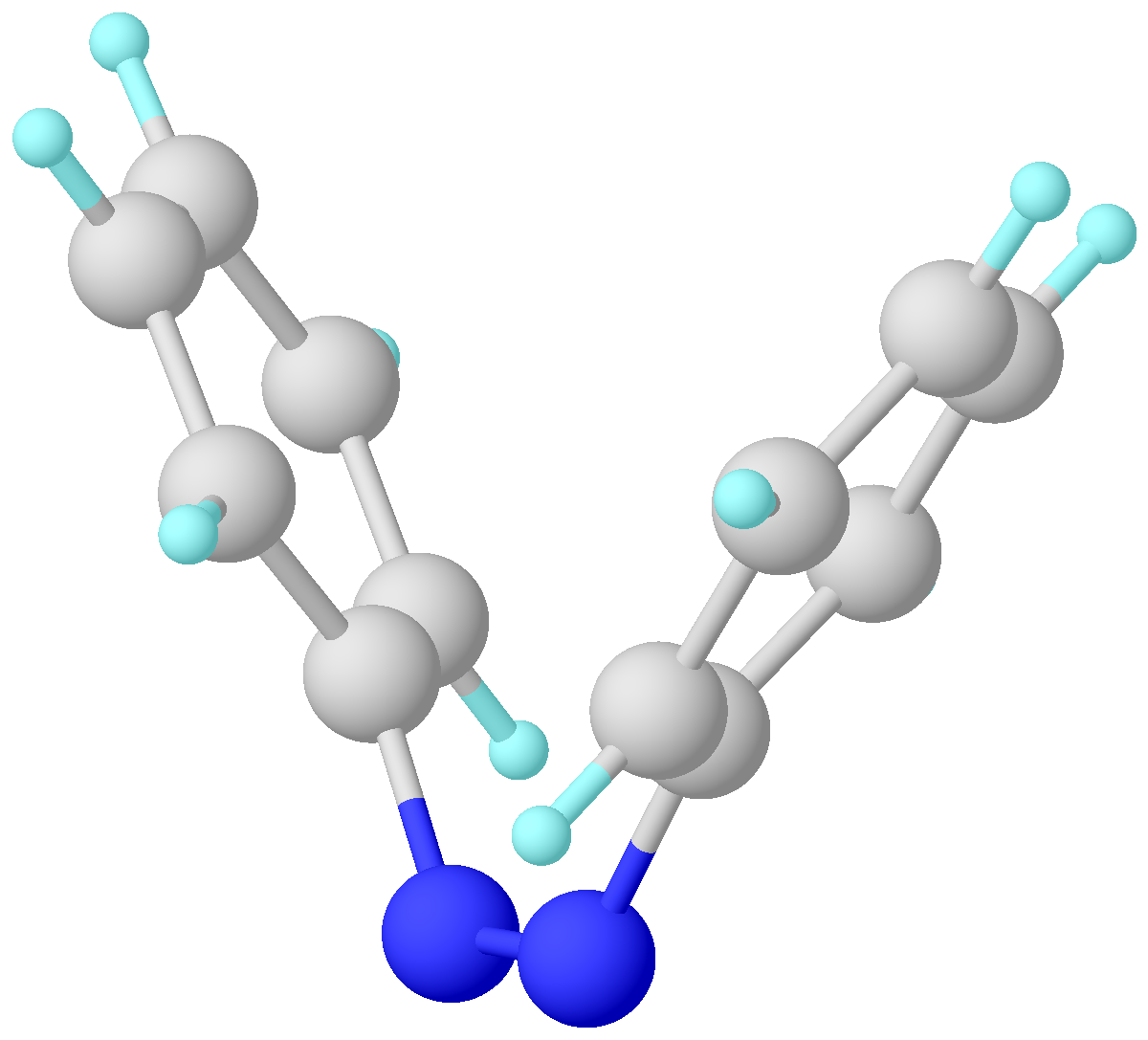
Azobenzene
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of similar compounds. These azo compounds are considered as derivatives of diazene (diimide), and are sometimes referred to as 'diazenes'. The diazenes absorb light strongly and are common dyes. Structure and synthesis ''trans''-Azobenzene is planar. The N-N distance is 1.189 Å. ''cis''-Azobenzene is nonplanar with a C-N=N-C dihedral angle of 173.5°. The N-N distance is 1.251 Å. Azobenzene was first described by Eilhard Mitscherlich in 1834. Yellowish-red crystalline flakes of azobenzene were obtained in 1856. Its original preparation is similar to the modern one. According to the 1856 method, nitrobenzene is reduced by iron filings in the presence of acetic acid. In the modern synthesis, zinc is the reductant in the presence of a base. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |