|
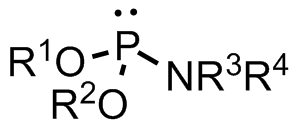
Phosphoramidite Structure
A phosphoramidite (RO)2PNR2 is a monoamide of a phosphite diester. The key feature of phosphoramidites is their markedly high reactivity towards nucleophiles catalyzed by weak acids ''e.c''., triethylammonium chloride or 1''H''-tetrazole. In these reactions, the incoming nucleophile replaces the NR2 moiety. Applications Nucleoside phosphoramidites Phosphoramidites derived from protected nucleosides are referred to as nucleoside phosphoramidites and are widely used in chemical synthesis of DNA, RNA, and other nucleic acids and their analogs. As ligands Certain phosphoramidites are also used as monodentate chiral ligands in asymmetric synthesis. A large group of such ligands is derived from the chiral diol BINOL and can be synthesised by reaction of BINOL with phosphorus trichloride to the chlorophosphite and then reaction with simple secondary amines. This type of ligand was first used in 1996 in an asymmetric copper-catalysed addition of dialkylzincs to enones. See also *Phos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoramidite Structure
A phosphoramidite (RO)2PNR2 is a monoamide of a phosphite diester. The key feature of phosphoramidites is their markedly high reactivity towards nucleophiles catalyzed by weak acids ''e.c''., triethylammonium chloride or 1''H''-tetrazole. In these reactions, the incoming nucleophile replaces the NR2 moiety. Applications Nucleoside phosphoramidites Phosphoramidites derived from protected nucleosides are referred to as nucleoside phosphoramidites and are widely used in chemical synthesis of DNA, RNA, and other nucleic acids and their analogs. As ligands Certain phosphoramidites are also used as monodentate chiral ligands in asymmetric synthesis. A large group of such ligands is derived from the chiral diol BINOL and can be synthesised by reaction of BINOL with phosphorus trichloride to the chlorophosphite and then reaction with simple secondary amines. This type of ligand was first used in 1996 in an asymmetric copper-catalysed addition of dialkylzincs to enones. See also *Phos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphite
The general structure of a phosphite ester showing the lone pairs on the P In organic chemistry, a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of an unobserved tautomer phosphorous acid, H3PO3, with the simplest example being trimethylphosphite, P(OCH3)3. Some phosphites can be considered esters of the dominant tautomer of phosphorous acid (HP(O)(OH)2). The simplest representative is dimethylphosphite with the formula HP(O)(OCH3)2. Both classes of phosphites are usually colorless liquids. Synthesis ;From PCl3 Phosphite esters are typically prepared by treating phosphorus trichloride with an alcohol. Depending on the synthetic details, this alcoholysis can give the diorganophosphites: :PCl3 + 3 C2H5OH → (C2H5O)2P(O)H + 2 HCl + C2H5Cl Alternatively, when the alcoholysis is conducted in the presence of proton acceptors, one obtains the C3-symmetric trialkoxy derivatives ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases. ''Nucleophilic'' describes the affinity of a nucleophile to bond with positively charged atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity. History The terms ''nucleophile'' and ''electrophile'' were introduced by Christopher Kelk Ingold in 1933, replacing the terms ''anionoid'' and ''cationoid' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrazole
Tetrazoles are a class of synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen atoms and one carbon atom. The name tetrazole also refers to the parent compound with formula CH2N4, of which three isomers can be formulated. Structure and bonding Three isomers of the parent tetrazole exist, differing in the position of the double bonds: 1''H''-, 2''H''-, and 5''H''-tetrazole. The 1''H''- and 2''H''- isomers are tautomers, with the equilibrium lying on the side of 1''H''-tetrazole in the solid phase. In the gas phase, 2''H''-tetrazole dominates. These isomers can be regarded as aromatic, with 6 π-electrons, while the 5''H''-isomer is nonaromatic. Synthesis 1''H''-Tetrazole was first prepared by the reaction of anhydrous hydrazoic acid and hydrogen cyanide under pressure. Treatment of organic nitriles with sodium azide in the presence of iodine or silica-supported sodium bisulfate as a heterogeneous catalyst enables an advantageous synthesis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleoside Phosphoramidite
Nucleoside phosphoramidites are derivatives of natural or synthetic nucleosides. They are used to synthesize oligonucleotides, relatively short fragments of nucleic acid and their analogs. Nucleoside phosphoramidites were first introduced in 1981 by Beaucage and Caruthers. To avoid undesired side reactions, reactive hydroxy and exocyclic amino groups present in natural or synthetic nucleosides are appropriately protected. As long as a nucleoside analog contains at least one hydroxy group, the use of the appropriate protecting strategy allows one to convert that to the respective phosphoramidite and to incorporate the latter into synthetic nucleic acids. To be incorporated in the middle of an oligonucleotide chain using phosphoramidite strategy, the nucleoside analog must possess two hydroxy groups or, less often, a hydroxy group and another nucleophilic group (amino or mercapto). Examples include, but are not limited to, alternative nucleotides, LNA, morpholino, nucleosides mod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oligonucleotide Synthesis
Oligonucleotide synthesis is the chemical synthesis of relatively short fragments of nucleic acids with defined chemical structure (sequence). The technique is extremely useful in current laboratory practice because it provides a rapid and inexpensive access to custom-made oligonucleotides of the desired sequence. Whereas enzymes synthesize DNA and RNA only in a 5' to 3' direction, chemical oligonucleotide synthesis does not have this limitation, although it is most often carried out in the opposite, 3' to 5' direction. Currently, the process is implemented as solid-phase synthesis using phosphoramidite method and phosphoramidite building blocks derived from protected 2'-deoxynucleosides ( dA, dC, dG, and T), ribonucleosides ( A, C, G, and U), or chemically modified nucleosides, e.g. LNA or BNA. To obtain the desired oligonucleotide, the building blocks are sequentially coupled to the growing oligonucleotide chain in the order required by the sequence of the product (see ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monodentate
In coordination chemistry, denticity () refers to the number of donor groups in a given ligand that bind to the central metal atom in a coordination complex. In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be monodentate (sometimes called unidentate). Ligands with more than one bonded atom are called polydentate or multidentate. The denticity of a ligand is described with the Greek letter κ ('kappa'). For example, κ6-EDTA describes an EDTA ligand that coordinates through 6 non-contiguous atoms. Denticity is different from hapticity because hapticity refers exclusively to ligands where the coordinating atoms are contiguous. In these cases the η ('eta') notation is used. Bridging ligands use the μ ('mu') notation. Classes Polydentate ligands are chelating agents and classified by their denticity. Some atoms cannot form the maximum possible number of bonds a ligand could make. In that case one or mor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chiral Ligand
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric (enantiomeric or diastereomeric) products in unequal amounts." Put more simply: it is the synthesis of a compound by a method that favors the formation of a specific enantiomer or diastereomer. Enantiomers are stereoisomers that have opposite configurations at every chiral center. Diastereomers are stereoisomers that differ at one or more chiral centers. Enantioselective synthesis is a key process in modern chemistry and is particularly important in the field of pharmaceuticals, as the different enantiomers or diastereomers of a molecule often have different biological activity. Overview Many of the building blocks of biological systems such as sugars and amino acids are produced exclusiv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Trichloride
Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts readily with water to release hydrogen chloride. History Phosphorus trichloride was first prepared in 1808 by the French chemists Joseph Louis Gay-Lussac and Louis Jacques Thénard by heating calomel (Hg2Cl2) with phosphorus. Later during the same year, the English chemist Humphry Davy produced phosphorus trichloride by burning phosphorus in chlorine gas. Preparation World production exceeds one-third of a million tonnes. Phosphorus trichloride is prepared industrially by the reaction of chlorine with white phosphorus, using phosphorus trichloride as the solvent. In this continuous process PCl3 is removed as it is formed in order to avoid the formation of PCl5. :P4 + 6 Cl2 → 4 PCl3 Structure and spectroscopy It has a trig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoramidate
Phosphoramidates (sometimes also called amidophosphates) are a class of phosphorus compounds structurally related to phosphates (or organophosphates) via the substitution of an OR for a NR2. They are derivatives of phosphoramidic acids O=P(OH)(NR2)2, O=P(OH)2(NR2). A phosphorodiamidate (or diamidophosphate) is a phosphate that has two of its OH groups substituted by NR2 groups to give a species with the general formula O=P(OH)(NH2)2. The substitution of all three OH groups gives the phosphoric triamides (O=P(NR2)3), which are commonly referred to as phosphoramides. Examples Two examples of natural phosphoramidates are phosphocreatine and the phosphoramidate formed when histidine residues in histidine kinases are phosphorylated. An example of a phosphorodiamidate is morpholino which is used in molecular biology. See also *Phosphoramidite A phosphoramidite (RO)2PNR2 is a monoamide of a phosphite diester. The key feature of phosphoramidites is their markedly high reactivity toward ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |