|
Phosphatidyl Ethanolamine Methyltransferase
Phosphatidylethanolamine N-methyltransferase (abbreviated PEMT) is a transferase enzyme () which catalysis, converts phosphatidylethanolamine (PE) to phosphatidylcholine (PC) in the liver. In humans it is encoded by the ''PEMT'' gene within the Smith–Magenis syndrome region on chromosome 17. While the CDP-choline pathway, in which choline obtained either by dietary consumption or by metabolism of choline-containing lipids is converted to PC, accounts for approximately 70% of PC biosynthesis in the liver, the PEMT pathway has been shown to have played a critical evolutionary role in providing PC during times of starvation. Furthermore, PC made via PEMT plays a wide range of physiological roles, utilized in choline synthesis, hepatocyte membrane structure, bile secretion, and very low-density lipoprotein (VLDL) secretion. Nomenclature Phosphatidylethanolamine N-methyltransferase is also known as lipid methyl transferase, LMTase, phosphatidylethanolamine methyltransferase, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PEMT Enzyme-Catalyzed Reaction
PEMT may refer to: * Phosphatidylethanolamine ''N''-methyltransferase, an enzyme encoded by the PEMT gene which synthesizes phosphatidylcholine * Polymorphic epithelial mucin, a mucin encoded by the MUC1 gene in humans * Post-edited machine translation, whereby humans amend machine-generated translation to achieve an acceptable final product {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phospholipid
Phospholipids, are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typically have omega-3 fatty acids EPA and DHA integrated as part of the phospholipid molecule. The phosphate group can be modified with simple organic molecules such as choline, ethanolamine or serine. Phospholipids are a key component of all cell membranes. They can form lipid bilayers because of their amphiphilic characteristic. In eukaryotes, cell membranes also contain another class of lipid, sterol, interspersed among the phospholipids. The combination provides fluidity in two dimensions combined with mechanical strength against rupture. Purified phospholipids are produced commercially and have found applications in nanotechnology and materials science. The first phospholipid identified in 1847 as such in biological tissues was lecith ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme Kinetics
Enzyme kinetics is the study of the rates of enzyme-catalysed chemical reactions. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction are investigated. Studying an enzyme's kinetics in this way can reveal the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled, and how a drug or a modifier ( inhibitor or activator) might affect the rate. An enzyme (E) is typically a protein molecule that promotes a reaction of another molecule, its substrate (S). This binds to the active site of the enzyme to produce an enzyme-substrate complex ES, and is transformed into an enzyme-product complex EP and from there to product P, via a transition state ES*. The series of steps is known as the mechanism: : E + S ⇄ ES ⇄ ES* ⇄ EP ⇄ E + P This example assumes the simplest case of a reaction with one substrate and one product. Such cases exist: for example, a mutase such as phosphoglucomutase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rate-determining Step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the corresponding rate equation (for comparison with the experimental rate law) is often simplified by using this approximation of the rate-determining step. In principle, the time evolution of the reactant and product concentrations can be determined from the set of simultaneous rate equations for the individual steps of the mechanism, one for each step. However, the analytical solution of these differential equations is not always easy, and in some cases numerical integration may even be required. The hypothesis of a single rate-determining step can greatly simplify the mathematics. In the simplest case the initial step is the slowest, and the overall rate is just the rate of the first step. Also, the rate equations for mechanisms with a s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
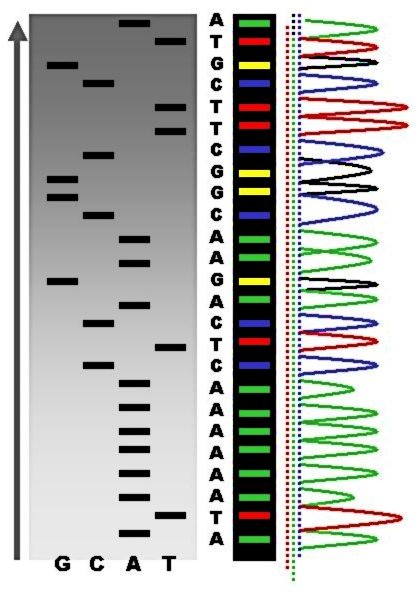
DNA Sequencing
DNA sequencing is the process of determining the nucleic acid sequence – the order of nucleotides in DNA. It includes any method or technology that is used to determine the order of the four bases: adenine, guanine, cytosine, and thymine. The advent of rapid DNA sequencing methods has greatly accelerated biological and medical research and discovery. Knowledge of DNA sequences has become indispensable for basic biological research, DNA Genographic Projects and in numerous applied fields such as medical diagnosis, biotechnology, forensic biology, virology and biological systematics. Comparing healthy and mutated DNA sequences can diagnose different diseases including various cancers, characterize antibody repertoire, and can be used to guide patient treatment. Having a quick way to sequence DNA allows for faster and more individualized medical care to be administered, and for more organisms to be identified and cataloged. The rapid speed of sequencing attained with modern D ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Sequencing
Protein sequencing is the practical process of determining the amino acid sequence of all or part of a protein or peptide. This may serve to identify the protein or characterize its post-translational modifications. Typically, partial sequencing of a protein provides sufficient information (one or more sequence tags) to identify it with reference to databases of protein sequences derived from the conceptual translation of genes. The two major direct methods of protein sequencing are mass spectrometry and Edman degradation using a protein sequenator (sequencer). Mass spectrometry methods are now the most widely used for protein sequencing and identification but Edman degradation remains a valuable tool for characterizing a protein's ''N''-terminus. Determining amino acid composition It is often desirable to know the unordered amino acid composition of a protein prior to attempting to find the ordered sequence, as this knowledge can be used to facilitate the discovery of errors i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. When present as a deprotonated catalytic residue, sometimes the symbol Cyz is used. The deprotonated form can generally be described by the symbol Cym as well. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. When used as a food additive, it has the E number E920. Cysteine is encoded by the codons UGU and UGC. The sulfur-containing amino acids cysteine and methionine are more easily oxidized than the other amino acids. Structure Like other amino acids (not as a residue of a protein), cysteine exists as a zwitterion. Cysteine has chirality in the older / notation based on homology to - and -glyceraldehyde. In the newer ''R''/''S'' system of designating chi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methionine
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical role in the metabolism and health of many species, including humans. It is encoded by the codon AUG. Methionine is also an important part of angiogenesis, the growth of new blood vessels. Supplementation may benefit those suffering from copper poisoning. Overconsumption of methionine, the methyl group donor in DNA methylation, is related to cancer growth in a number of studies. Methionine was first isolated in 1921 by John Howard Mueller. Biochemical details Methionine (abbreviated as Met or M; encoded by the codon AUG) is an α-amino acid that is used in the biosynthesis of proteins. It contains a carboxyl group (which is in the deprotonated −COO− form under biological pH conditions), an amino group (which is in the protonated fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homocysteine
Homocysteine is a non-proteinogenic α-amino acid. It is a homologue of the amino acid cysteine, differing by an additional methylene bridge (-CH2-). It is biosynthesized from methionine by the removal of its terminal Cε methyl group. In the body, homocysteine can be recycled into methionine or converted into cysteine with the aid of certain B-vitamins. High levels of homocysteine in the blood (hyperhomocysteinemia) is regarded as a marker of cardiovascular disease, likely working through atherogenesis, which can result in ischemic injury. Therefore, hyperhomocysteinemia is a possible risk factor for coronary artery disease. Coronary artery disease occurs when an atherosclerotic plaque blocks blood flow to the coronary arteries, which supply the heart with oxygenated blood. Hyperhomocysteinemia has been correlated with the occurrence of blood clots, heart attacks, and strokes, although it is unclear whether hyperhomocysteinemia is an independent risk factor for these conditi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arachidonic Acid
Arachidonic acid (AA, sometimes ARA) is a polyunsaturated omega-6 fatty acid 20:4(ω-6), or 20:4(5,8,11,14). It is structurally related to the saturated arachidic acid found in cupuaçu butter. Its name derives from the New Latin word ''arachis'' (peanut), but peanut oil does not contain any arachidonic acid. Chemistry In chemical structure, arachidonic acid is a carboxylic acid with a 20-carbon chain and four ''cis''-double bonds; the first double bond is located at the sixth carbon from the omega end. Some chemistry sources define 'arachidonic acid' to designate any of the eicosatetraenoic acids. However, almost all writings in biology, medicine, and nutrition limit the term to ''all cis''-5,8,11,14-eicosatetraenoic acid. Biology Arachidonic acid is a polyunsaturated fatty acid present in the phospholipids (especially phosphatidylethanolamine, phosphatidylcholine, and phosphatidylinositides) of membranes of the body's cells, and is abundant in the brain, muscles, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyunsaturated Fat
Polyunsaturated fats are fats in which the constituent hydrocarbon chain possesses two or more carbon–carbon double bonds. Polyunsaturated fat can be found mostly in nuts, seeds, fish, seed oils, and oysters. "Unsaturated" refers to the fact that the molecules contain less than the maximum amount of hydrogen (if there were no double bonds). These materials exist as ''cis'' or ''trans'' isomers depending on the geometry of the double bond. Saturated fats have hydrocarbon chains which can be most readily aligned. The hydrocarbon chains in trans fats align more readily than those in cis fats, but less well than those in saturated fats. In general, this means that the melting points of fats increase from cis to trans unsaturated and then to saturated. See the section about the chemical structure of fats for more information. The position of the carbon-carbon double bonds in carboxylic acid chains in fats is designated by Greek letters. The carbon atom closest to the carboxyl gr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
De Novo Synthesis
In chemistry, ''de novo'' synthesis () refers to the synthesis of complex molecules from simple molecules such as sugars or amino acids, as opposed to recycling after partial degradation. For example, nucleotides are not needed in the diet as they can be constructed from small precursor molecules such as formate and aspartate. Methionine, on the other hand, is needed in the diet because while it can be degraded to and then regenerated from homocysteine, it cannot be synthesized ''de novo''. Nucleotide ''De novo'' pathways of nucleotides do not use free bases: adenine (abbreviated as A), guanine (G), cytosine (C), thymine (T), or uracil (U). The purine ring is built up one atom or a few atoms at a time and attached to ribose throughout the process. Pyrimidine ring is synthesized as orotate and attached to ribose phosphate and later converted to common pyrimidine nucleotides. Cholesterol Cholesterol is an essential structural component of animal cell membranes. Cholestero ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |