|
Phenol Ether
In chemistry, a phenol ether (or aromatic ether) is an organic compound derived from phenol (C6H5OH), where the hydroxyl (-OH) group is substituted with an alkoxy (-OR) group. Usually phenol ethers are synthesized through the condensation of phenol and an organic alcohol; however, other known reactions regarding the synthesis of ethers can be applied to phenol ethers as well. Anisole (C6H5OCH3) is the simplest phenol ether, and is a versatile precursor for perfumes and pharmaceuticals. Vanillin and ethylvanillin are phenol ether derivatives commonly utilized in vanilla flavorings and fragrances, while diphenyl ether is commonly used as a synthetic geranium fragrance. Phenol ethers are part of the chemical structure of a variety of medications, including quinine, an antimalarial drug, and dextromethorphan, an over-the-counter cough suppressant. Nomenclature Phenol ethers follow the same nomenclature of regular ethers; ethers have the structure R-O-R’, but phenol ethers require t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It is commonly used as a solvent in laboratories and as a starting fluid for some engines. It was formerly used as a general anesthetic, until non-flammable drugs were developed, such as halothane. It has been used as a recreational drug to cause intoxication. Production Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid catalysts and can be adjusted to make more ether if the need arises. Vapor-phase dehydration of ethanol over some alumina catalysts can give diethyl ether yields of up to 95%. Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis. Ethanol is mixed with a stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipinski's Rule Of Five
Lipinski's rule of five, also known as Pfizer's rule of five or simply the rule of five (RO5), is a rule of thumb to evaluate druglikeness or determine if a chemical compound with a certain pharmacological or biological activity has chemical properties and physical properties that would make it a likely orally active drug in humans. The rule was formulated by Christopher A. Lipinski in 1997, based on the observation that most orally administered drugs are relatively small and moderately lipophilic molecules. The rule describes molecular properties important for a drug's pharmacokinetics in the human body, including their absorption, distribution, metabolism, and excretion ("ADME"). However, the rule does not predict if a compound is pharmacologically active. The rule is important to keep in mind during drug discovery when a pharmacologically active lead structure is optimized step-wise to increase the activity and selectivity of the compound as well as to ensure drug-like phy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bond Acceptor
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted , where the solid line denotes a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are the second-row elements nitrogen (N), oxygen (O), and fluorine (F). Hydrogen bonds can be intermolecular (occurring between separate molecules) or intramolecular (occurring among parts of the same molecule). The energy of a hydrogen bond depends on the geometry, the environment, and the nature of the specific donor and acceptor atoms and can vary between 1 and 40 kcal/mol. This makes them somewhat stronger than a van der Waals interaction, and weaker than fully covalent or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(II) Oxide
Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It is a product of copper mining and the precursor to many other copper-containing products and chemical compounds. Production It is produced on a large scale by pyrometallurgy, as one stage in extracting copper from its ores. The ores are treated with an aqueous mixture of ammonium carbonate, ammonia, and oxygen to give copper(I) and copper(II) ammine complexes, which are extracted from the solids. These complexes are decomposed with steam to give CuO. It can be formed by heating copper in air at around 300–800°C: : 2 Cu + O2 → 2 CuO For laboratory uses, pure copper(II) oxide is better prepared by heating copper(II) nitrate, copper(II) hydroxide, or basic copper(II) carbonate: : 2 Cu(NO3)2(s) → 2 CuO(s) + 4 NO2(g) + O2(g) (180° ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ullmann Condensation
The Ullmann condensation or Ullmann-type reaction is the copper-promoted conversion of aryl halides to aryl ethers, aryl thioethers, aryl nitriles, and aryl amines. These reactions are examples of cross-coupling reactions. Ullmann-type reactions are comparable to Buchwald–Hartwig reactions but usually require higher temperatures. Traditionally these reaction requires high-boiling polar solvents such as ''N''-methylpyrrolidone, nitrobenzene, or dimethylformamide and high temperatures (often in excess of 210 °C) with stoichiometric amounts of copper. Aryl halide were required to be activated by electron-withdrawing groups. Traditional Ullmann style reactions used "activated" copper powder, e.g. prepared in situ by the reduction of copper sulfate by zinc metal in hot water. The methodology improved with the introduction of soluble copper catalysts supported by diamines and acetylacetonate ligands. Ullmann ether synthesis: C-O coupling Illustrative of the traditional Ullm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
E2 Elimination
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 reaction. The numbers refer not to the number of steps in the mechanism, but rather to the kinetics of the reaction: E2 is bimolecular (second-order) while E1 is unimolecular (first-order). In cases where the molecule is able to stabilize an anion but possesses a poor leaving group, a third type of reaction, E1CB, exists. Finally, the pyrolysis of xanthate and acetate esters proceed through an "internal" elimination mechanism, the Ei mechanism. E2 mechanism The E2 mechanism, where E2 stands for bimolecular elimination, involves a one-step mechanism in which ''carbon-hydrogen'' and ''carbon-halogen'' bonds break to form a double bond (''C=C Pi bond''). The specifics of the reaction are as follows: * E2 is a single step elimination, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
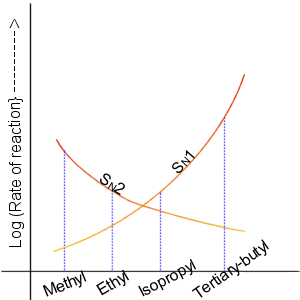
Nucleophilic Substitution
In chemistry, a nucleophilic substitution is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The molecule that contains the electrophile and the leaving functional group is called the substrate. The most general form of the reaction may be given as the following: :\text\mathbf + \ce + \text\mathbf The electron pair (:) from the nucleophile (Nuc) attacks the substrate () and bonds with it. Simultaneously, the leaving group (LG) departs with an electron pair. The principal product in this case is . The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. An example of nucleophilic substitution is the hydrolysis of an alkyl bromide, R-Br under basic conditions, where the attacking nucleophile is hydroxyl () and the leaving group is bromide (). :R-Br + OH- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenolates
Phenolates (also called phenoxides) are anions, salts, and esters of phenols. They may be formed by reaction of phenols with strong base. Properties Alkali metal phenolates, such as sodium phenolate hydrolyze in aqueous solution to form basic solutions. At pH = 10, phenol and phenolate are in approximately 1:1 proportions. Phenolate anions are enolates. As such, they react as nucleophiles at both oxygen and carbon positions. In general, reaction at oxygen occurs under kinetic control, whereas reaction at carbon occurs under thermodynamic control. Uses Alkyl aryl ethers can be synthesized through the Williamson ether synthesis by treating sodium phenolate with an alkyl halide: :C6H5ONa + CH3I → C6H5OCH3 + NaI :C6H5ONa + (CH3O)2SO2 → C6H5OCH3 + (CH3O)SO3Na Production of salicylic acid Salicylic acid is produced in the Kolbe–Schmitt reaction between carbon dioxide and sodium phenolate. : See also * Sodium phenolate Sodium phenoxide (sodium phenolate) is an organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |