|
Pertussis Toxin
Pertussis toxin (PT) is a protein-based AB5-type exotoxin produced by the bacterium '' Bordetella pertussis'', which causes whooping cough. PT is involved in the colonization of the respiratory tract and the establishment of infection. Research suggests PT may have a therapeutic role in treating a number of common human ailments, including hypertension, viral infection, and autoimmunity. History PT clearly plays a central role in the pathogenesis of pertussis although this was discovered only in the early 1980s. The appearance of pertussis is quite recent, compared with other epidemic infectious diseases. The earliest mention of pertussis, or whooping cough, is of an outbreak in Paris in 1414. This was published in Moulton's The Mirror of Health, in 1640. Another epidemic of pertussis took place in Paris in 1578 and was described by a contemporary observer, Guillaume de Baillou. Pertussis was well known throughout Europe by the middle of the 18th century. Jules Bordet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AB5 Toxin
The AB5 toxins are six-component protein complexes secreted by certain pathogenic bacteria known to cause human diseases such as cholera, dysentery, and hemolytic–uremic syndrome. One component is known as the A subunit, and the remaining five components are B subunits. All of these toxins share a similar structure and mechanism for entering targeted host cells. The B subunit is responsible for binding to receptors to open up a pathway for the A subunit to enter the cell. The A subunit is then able to use its catalytic machinery to take over the host cell's regular functions. Families There are four main families of the AB5 toxin. These families are characterized by the sequence of their A (catalytic) subunit, as well as their catalytic activity. Cholera toxin This family is also known as Ct or Ctx, and also includes the heat-labile enterotoxin, known as LT. Cholera toxin's discovery is credited by many to Dr. Sambhu Nath De. He conducted his research in Calcutta ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endoplasmic Reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa. The two types of ER share many of the same proteins and engage in certain common activities such as the synthesis of certain lipids and cholesterol. Different types of cells contain different ratios of the two types of ER depending on the activities of the cell. RER is found mainly toward the nucleus of cell and SER towards the cell membrane or pl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemotaxis
Chemotaxis (from '' chemo-'' + '' taxis'') is the movement of an organism or entity in response to a chemical stimulus. Somatic cell A somatic cell (from Ancient Greek σῶμα ''sôma'', meaning "body"), or vegetal cell, is any biological cell forming the body of a multicellular organism other than a gamete, germ cell, gametocyte or undifferentiated stem cell. Such cells compo ...s, bacteria, and other single-cell organism, single-cell or multicellular organisms direct their movements according to certain chemicals in their environment. This is important for bacteria to find food (e.g., glucose) by swimming toward the highest concentration of food molecules, or to flee from poisons (e.g., phenol). In multicellular organisms, chemotaxis is critical to early development (e.g., movement of sperm towards the egg during fertilization) and development (e.g., migration of neurons or lymphocytes) as well as in normal function and health (e.g., migration of White blood cell, leukocytes d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemokine
Chemokines (), or chemotactic cytokines, are a family of small cytokines or Cell signaling, signaling proteins secreted by Cell (biology), cells that induce directional movement of leukocytes, as well as other cell types, including endothelial and epithelial cells. In addition to playing a major role in the activation of host immune responses, chemokines are important for biological processes, including morphogenesis and wound healing, as well as in the pathogenesis of diseases like cancers. Cytokine proteins are classified as chemokines according to behavior and structural characteristics. In addition to being known for mediating chemotaxis, chemokines are all approximately 8-10 kilodaltons in mass and have four cysteine residues in conserved locations that are key to forming their 3-dimensional shape. These proteins have historically been known under several other names including the ''SIS family of cytokines'', ''SIG family of cytokines'', ''SCY family of cytokines'', ''Plate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macrophages
Macrophages (abbreviated as M φ, MΦ or MP) ( el, large eaters, from Greek ''μακρός'' (') = large, ''φαγεῖν'' (') = to eat) are a type of white blood cell of the immune system that engulfs and digests pathogens, such as cancer cells, microbes, cellular debris, and foreign substances, which do not have proteins that are specific to healthy body cells on their surface. The process is called phagocytosis, which acts to defend the host against infection and injury. These large phagocytes are found in essentially all tissues, where they patrol for potential pathogens by amoeboid movement. They take various forms (with various names) throughout the body (e.g., histiocytes, Kupffer cells, alveolar macrophages, microglia, and others), but all are part of the mononuclear phagocyte system. Besides phagocytosis, they play a critical role in nonspecific defense ( innate immunity) and also help initiate specific defense mechanisms ( adaptive immunity) by recruiting oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutrophils
Neutrophils (also known as neutrocytes or heterophils) are the most abundant type of granulocytes and make up 40% to 70% of all white blood cells in humans. They form an essential part of the innate immune system, with their functions varying in different animals. They are formed from stem cells in the bone marrow and differentiated into subpopulations of neutrophil-killers and neutrophil-cagers. They are short-lived and highly mobile, as they can enter parts of tissue where other cells/molecules cannot. Neutrophils may be subdivided into segmented neutrophils and banded neutrophils (or bands). They form part of the polymorphonuclear cells family (PMNs) together with basophils and eosinophils. The name ''neutrophil'' derives from staining characteristics on hematoxylin and eosin ( H&E) histological or cytological preparations. Whereas basophilic white blood cells stain dark blue and eosinophilic white blood cells stain bright red, neutrophils stain a neutral pink. No ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypoglycemia
Hypoglycemia, also called low blood sugar, is a fall in blood sugar to levels below normal, typically below 70 mg/dL (3.9 mmol/L). Whipple's triad is used to properly identify hypoglycemic episodes. It is defined as blood glucose below 70 mg/dL (3.9 mmol/L), symptoms associated with hypoglycemia, and resolution of symptoms when blood sugar returns to normal. Hypoglycemia may result in headache, tiredness, clumsiness, trouble talking, confusion, fast heart rate, sweating, shakiness, nervousness, hunger, loss of consciousness, seizures, or death. Symptoms typically come on quickly. The most common cause of hypoglycemia is medications used to treat diabetes such as insulin, sulfonylureas, and biguanides. Risk is greater in diabetics who have eaten less than usual, recently exercised, or consumed alcohol. Other causes of hypoglycemia include severe illness, sepsis, kidney failure, liver disease, hormone deficiency, tumors such as insulinomas or n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insulin
Insulin (, from Latin ''insula'', 'island') is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the ''INS'' gene. It is considered to be the main anabolic hormone of the body. It regulates the metabolism of carbohydrates, fats and protein by promoting the absorption of glucose from the blood into liver, fat and skeletal muscle cells. In these tissues the absorbed glucose is converted into either glycogen via glycogenesis or fats ( triglycerides) via lipogenesis, or, in the case of the liver, into both. Glucose production and secretion by the liver is strongly inhibited by high concentrations of insulin in the blood. Circulating insulin also affects the synthesis of proteins in a wide variety of tissues. It is therefore an anabolic hormone, promoting the conversion of small molecules in the blood into large molecules inside the cells. Low insulin levels in the blood have the opposite effect by promoting widespread catabolism, espe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (the extracellular space). The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols (a lipid component) interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of cells and organelles, being selectively permeable to ion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
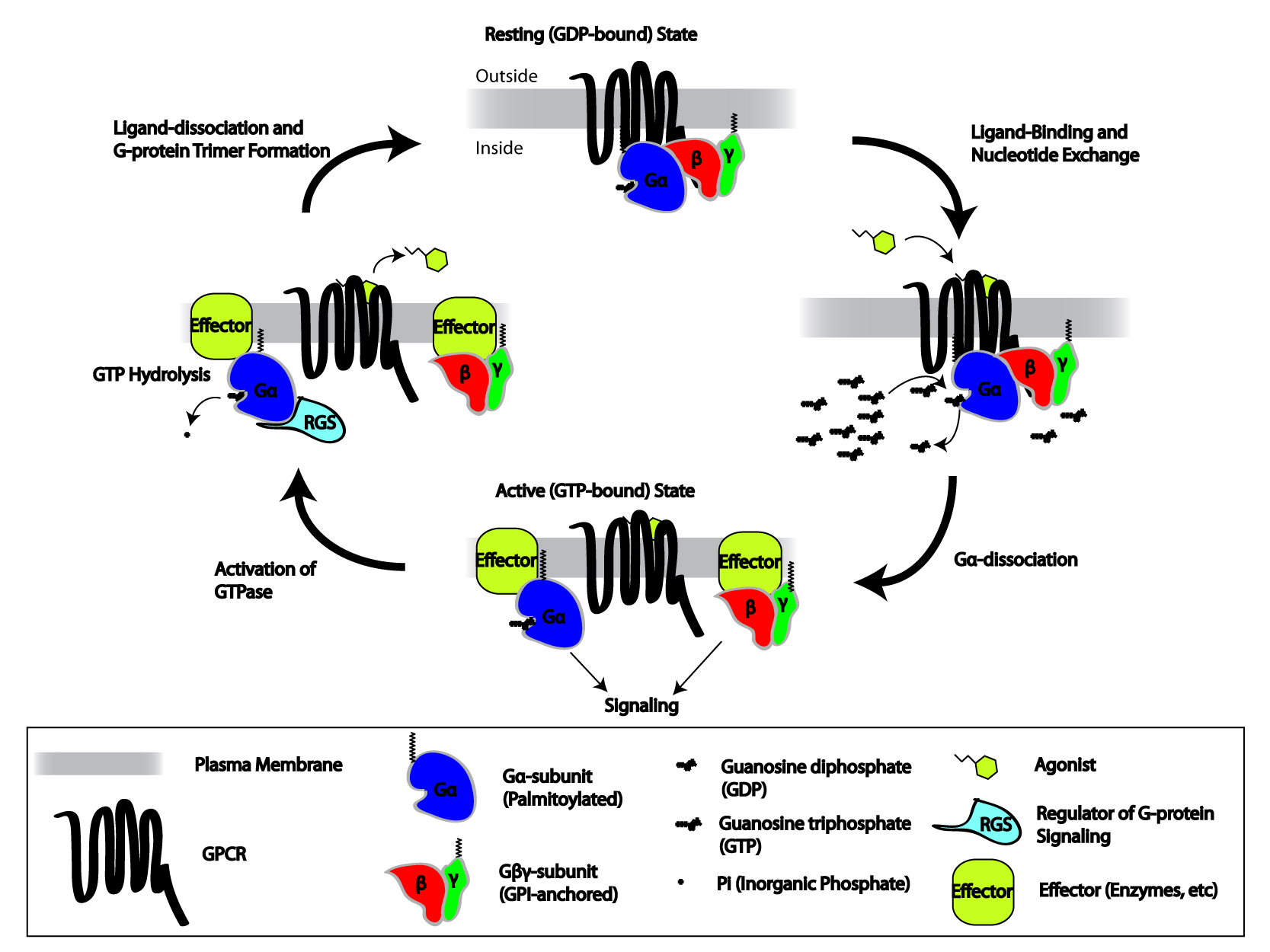
G Protein-coupled Receptor
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. Coupling with G proteins, they are called seven-transmembrane receptors because they pass through the cell membrane seven times. Text was copied from this source, which is available under Attribution 2.5 Generic (CC BY 2.5) license. Ligands can bind either to extracellular N-terminus and loops (e.g. glutamate receptors) or to the binding site within transmembrane helices (Rhodopsin-like family). They are all activated by agonists although a spontaneous auto-activation of an empty receptor can also be observed. G protein-coupled receptors are found only in eukaryotes, including yeast, choanoflagellates, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterotrimeric G Protein
Heterotrimeric G protein, also sometimes referred to as the ''"large" G proteins'' (as opposed to the subclass of smaller, monomeric small GTPases) are membrane-associated G proteins that form a heterotrimeric complex. The biggest non-structural difference between heterotrimeric and monomeric G protein is that heterotrimeric proteins bind to their cell-surface receptors, called G protein-coupled receptors, directly. These G proteins are made up of ''alpha'' (α), ''beta'' (β) and ''gamma'' (γ) subunits. The alpha subunit is attached to either a GTP or GDP, which serves as an on-off switch for the activation of G-protein. When ligands bind a GPCR, the GPCR acquires GEF ( guanine nucleotide exchange factor) ability, which activates the G-protein by exchanging the GDP on the ''alpha'' subunit to GTP. The binding of GTP to the ''alpha'' subunit results in a structural change and its dissociation from the rest of the G-protein. Generally, the ''alpha'' subunit binds membrane-boun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gi Alpha Subunit
Gi protein alpha subunit is a family of heterotrimeric G protein alpha subunits. This family is also commonly called the Gi/o (Gi /Go ) family or Gi/o/z/t family to include closely related family members. G alpha subunits may be referred to as Gi alpha, Gαi, or Giα. Family members There are four distinct subtypes of alpha subunits in the Gi/o/z/t alpha subunit family that define four families of heterotrimeric G proteins: * Gi proteins: Gi1α, Gi2α, and Gi3α * Go protein: Goα (in mouse there is alternative splicing to generate Go1α and Go2α) * Gz protein: Gzα * Transducins (Gt proteins): Gt1α, Gt2α, Gt3α Giα proteins Gi1α Gi1α is encoded by the gene GNAI1. Gi2α Gi2α is encoded by the gene GNAI2. Gi3α Gi3α is encoded by the gene GNAI3. Goα protein Go1α is encoded by the gene GNAO1. Gzα protein Gzα is encoded by the gene GNAZ. Transducin proteins Gt1α Transducin/Gt1α is encoded by the gene GNAT1. Gt2α Transduc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |