|
Pentafluorophenyl Esters
Pentafluorophenyl (PFP) esters are chemical compounds with the generic formula RC(O)OC6F5. They are active esters derived from pentafluorophenol (HOC6F5). PFP esters are useful for attaching fluorophores such as fluorescein or haptens to primary amines in biomolecules. They also are valuable in laboratory peptide synthesis In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl .... Pentafluorophenyl esters produce amide bonds as effectively as succinimidyl esters and various similar agents do, but PFP esters are particularly useful because they are less susceptible to spontaneous hydrolysis during conjugation reactions. References {{Reflist Esters Pentafluorophenyl compounds Phenol esters Reagents for organic chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Active Ester
In organic chemistry, an active ester is an ester functional group that is highly susceptible toward nucleophilic attack. Activation can be imparted by modifications of the acyl or the alkoxy components of a normal ester, say ethyl acetate. Typical modifications call for electronegative substituents. Active esters are employed in both synthetic and biological chemistry. Reactivity Active esters are mainly used as acylating agents. They undergo the same reactions as their unactivated analogues but do so more rapidly. They are prone to hydrolysis, for example. Of great interest is the enhanced reactivity of active esters toward amines to give amides. Examples Thioesters are prominent active esters, as illustrated by the esters of coenzyme A. In synthetic chemistry, active esters include derivatives of nitrophenols and pentafluorophenol. Active esters are often used in peptide synthesis, e.g., N-hydroxysuccinimide, hydroxybenzotriazole. Active esters of acrylic acid are precurs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentafluorophenol
Pentafluorophenol is the organofluorine compound (specifically a fluoroalcohol) with the formula C6F5OH. This is the perfluorinated analogue of phenol. It is a white odorless solid that melts just above room temperature. With a pKa of 5.5, it is one of the most acidic phenols. Uses Pentafluorophenol is used to prepare pentafluorophenyl esters, which are active esters useful in peptide synthesis In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl .... Environmental hazards Pentafluorophenol is considered hazardous because of oral, dermal and inhalation toxicity and because it causes "severe skin burns and eye damage." References {{Reflist Fluoroarenes Perfluorinated compounds Phenols ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
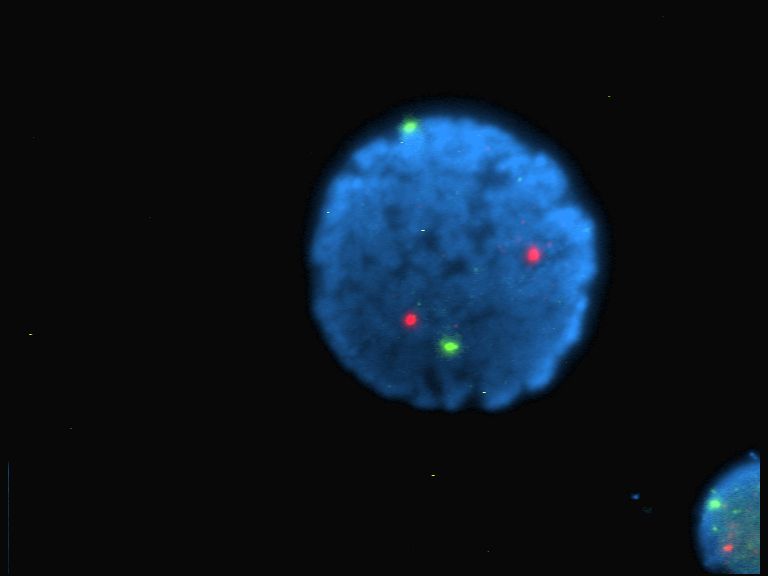
Fluorophore
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds. Fluorophores are sometimes used alone, as a tracer in fluids, as a dye for staining of certain structures, as a substrate of enzymes, or as a probe or indicator (when its fluorescence is affected by environmental aspects such as polarity or ions). More generally they are covalently bonded to a macromolecule, serving as a marker (or dye, or tag, or reporter) for affine or bioactive reagents (antibodies, peptides, nucleic acids). Fluorophores are notably used to stain tissues, cells, or materials in a variety of analytical methods, i.e., fluorescent imaging and spectroscopy. Fluorescein, via its amine-reactive isothiocyanate derivative fluorescein isothiocyanate (FITC), has been one of the most popular fluorophores. Fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescein
Fluorescein is an organic compound and dye based on the xanthene tricyclic structural motif, formally belonging to triarylmethine dyes family. It is available as a dark orange/red powder slightly soluble in water and alcohol. It is widely used as a fluorescent tracer for many applications. The color of its aqueous solutions is green by reflection and orange by transmission (its spectral properties are dependent on pH of the solution), as can be noticed in bubble levels, for example, in which fluorescein is added as a colorant to the alcohol filling the tube in order to increase the visibility of the air bubble contained within (thus enhancing the precision of the instrument). More concentrated solutions of fluorescein can even appear red (because under these conditions nearly all incident emission is re-absorbed by the solution). It is on the World Health Organization's List of Essential Medicines. Uses Fluorescein sodium, the sodium salt of fluorescein, is used extensi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hapten
In immunology, haptens are small molecules that elicit an immune response only when attached to a large carrier such as a protein; the carrier may be one that also does not elicit an immune response by itself (in general, only large molecules, infectious agents, or insoluble foreign matter can elicit an immune response in the body). Once the body has generated antibodies to a hapten-carrier adduct, the small-molecule hapten may also be able to bind to the antibody, but it will usually not initiate an immune response; usually only the hapten-carrier adduct can do this. Sometimes the small-molecule hapten can even block immune response to the hapten-carrier adduct by preventing the adduct from binding to the antibody, a process called ''hapten inhibition''. The mechanisms of absence of immune response may vary and involve complex immunological mechanisms, but can include absent or insufficient co-stimulatory signals from antigen-presenting cells. Haptens have been used to study al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide Synthesis
In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains. Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide (C-terminus), and proceeds toward the amino-terminus (N-terminus). Protein biosynthesis (long peptides) in living organisms occurs in the opposite direction. The chemical synthesis of peptides can be carried out using classical solution-phase techniques, although these have been replaced in most research and development settings by solid-phase methods (see below). Solution-phase synthesis retains its usefulness in large-scale production of peptides for industrial purposes how ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Esters
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature Etymology The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol Esters
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 billion kg/year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical drugs. Properties Phenol is an organic compound appreciably soluble in water, with about 84.2 g dissolving in 1000 mL (0.895 M). Homogeneous mixtures of phenol and water at phenol to wate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |