|
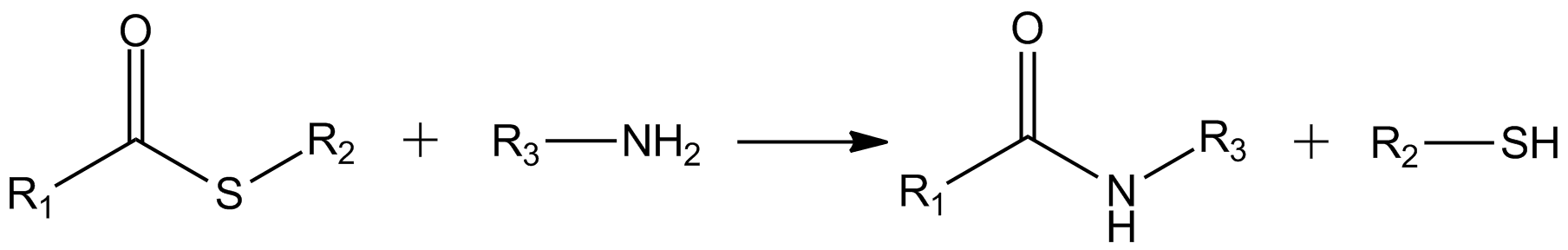
Active Ester
In organic chemistry, an active ester is an ester functional group that is highly susceptible toward nucleophilic attack. Activation can be imparted by modifications of the acyl or the alkoxy components of a normal ester, say ethyl acetate. Typical modifications call for electronegative substituents. Active esters are employed in both synthetic and biological chemistry. Reactivity Active esters are mainly used as acylating agents. They undergo the same reactions as their unactivated analogues but do so more rapidly. They are prone to hydrolysis, for example. Of great interest is the enhanced reactivity of active esters toward amines to give amides. Examples Thioesters are prominent active esters, as illustrated by the esters of coenzyme A. In synthetic chemistry, active esters include derivatives of nitrophenols and pentafluorophenol. Active esters are often used in peptide synthesis, e.g., N-hydroxysuccinimide, hydroxybenzotriazole. Active esters of acrylic acid are precur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrophenol
Nitrophenols are compounds of the formula HOC6H5−x(NO2)x. The conjugate bases are called nitrophenolates. Nitrophenols are more acidic than phenol itself. Mono-nitrophenols with the formula HOC6H4NO2. Three isomeric nitrophenols exist: * ''o''-Nitrophenol (2-nitrophenol; OH and NO2 groups are neighboring; CAS number: 88-75-5), a yellow crystalline solid (m.p. 46 °C). * ''m''-Nitrophenol (3-nitrophenol, CAS number: 554-84-7), a yellow solid (m.p. 97 °C) and precursor to the drug mesalazine (5-aminosalicylic acid). * ''p''-Nitrophenol (4-nitrophenol, CAS number: 100-02-7), yellow crystals (m.p. 114 °C). It is a precursor to the rice herbicide fluorodifen, the pesticide parathion, and the human analgesic paracetamol (also known as acetaminophen). The mononitrated phenols are often hydrogenated to the corresponding aminophenols that are also useful industrially. Di- and trinitrophenols * 2,4-Dinitrophenol (m.p. 83 °C) is a moderately strong acid (pKa = 4.8 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylating Agent
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These terms are commonly used in chemistry, biochemistry, soil science, and the biological sciences. In biological systems, methylation is catalyzed by enzymes; such methylation can be involved in modification of heavy metals, regulation of gene expression, regulation of protein function, and RNA processing. In vitro methylation of tissue samples is also one method for reducing certain histological staining artifacts. The reverse of methylation is demethylation. In biology In biological systems, methylation is accomplished by enzymes. Methylation can modify heavy metals, regulate gene expression, RNA processing and protein function. It has been recognized as a key process underlying epigenetics. Methanogenesis Methanogenesis, the process t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylsulfate
Dimethyl sulfate (DMS) is a chemical compound with formula (CH3O)2SO2. As the diester of methanol and sulfuric acid, its formula is often written as ( CH3)2 SO4 or Me2SO4, where CH3 or Me is methyl. Me2SO4 is mainly used as a methylating agent in organic synthesis. Me2SO4 is a colourless oily liquid with a slight onion-like odour (although smelling it would represent significant exposure). Like all strong alkylating agents, Me2SO4 is extremely toxic. Its use as a laboratory reagent has been superseded to some extent by methyl triflate, CF3SO3CH3, the methyl ester of trifluoromethanesulfonic acid. History Dimethyl sulfate was discovered in the early 19th century in an impure form. J. P. Claesson later extensively studied its preparation. It was investigated as a candidate for possible use chemical warfare in World War I in 75% to 25% mixture with methyl chlorosulfonate (CH3ClO3S) called "C-stoff" in Germany, or with chlorosulfonic acid called "Rationite" in France. P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acrylic Acid
Acrylic acid (IUPAC: propenoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols, ethers, and chloroform. More than a million tons are produced annually. History The word "acrylic" was coined in 1843, for a chemical derivative of acrolein, an acrid-smelling oil derived from glycerol. Production Acrylic acid is produced by oxidation of propylene, which is a byproduct of the production of ethylene and gasoline: : 2 CH2=CHCH3 + 3 O2 → 2 CH2=CHCO2H + 2 H2O Historical methods Because acrylic acid and its esters have long been valued commercially, many other methods have been developed. Most have been abandoned for economic or environmental reasons. An early method was the hydrocarboxylation of acetylene (" Reppe chemistry"): : This method req ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxybenzotriazole
Hydroxybenzotriazole (abbreviated HOBt) is an organic compound that is a derivative of benzotriazole. It is a white crystalline powder, which as a commercial product contains some water (~11.7% wt as the HOBt monohydrate crystal). Anhydrous HOBt is explosive. It is mainly used to suppress the racemization of single-enantiomer chiral molecules and to improve the efficiency of peptide synthesis. Use in peptide synthesis Automated peptide synthesis involves the condensation of the amino group of protected amino acids with the activated ester. HOBt is used to produce such activated esters. These esters are insoluble (like the N-hydroxysuccinimide esters) and react with amines at ambient temperature to give amides. HOBt is also used for the synthesis of amides from carboxylic acids aside from amino acids. These substrates may not be convertible to the acyl chlorides. For instance amide derivatives of ionophoric antibiotics have been prepared in this way.{{cite journal, last=Łowi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide Synthesis
In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains. Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide (C-terminus), and proceeds toward the amino-terminus (N-terminus). Protein biosynthesis (long peptides) in living organisms occurs in the opposite direction. The chemical synthesis of peptides can be carried out using classical solution-phase techniques, although these have been replaced in most research and development settings by solid-phase methods (see below). Solution-phase synthesis retains its usefulness in large-scale production of peptides for industrial purposes how ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentafluorophenol
Pentafluorophenol is the organofluorine compound (specifically a fluoroalcohol) with the formula C6F5OH. This is the perfluorinated analogue of phenol. It is a white odorless solid that melts just above room temperature. With a pKa of 5.5, it is one of the most acidic phenols. Uses Pentafluorophenol is used to prepare pentafluorophenyl esters, which are active esters useful in peptide synthesis In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxy .... Environmental hazards Pentafluorophenol is considered hazardous because of oral, dermal and inhalation toxicity and because it causes "severe skin burns and eye damage." References {{Reflist Fluoroarenes Perfluorinated compounds Phenols ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coenzyme A
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All genomes sequenced to date encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it (or a thioester) as a substrate. In humans, CoA biosynthesis requires cysteine, pantothenate (vitamin B5), and adenosine triphosphate (ATP). In its acetyl form, coenzyme A is a highly versatile molecule, serving metabolic functions in both the anabolic and catabolic pathways. Acetyl-CoA is utilised in the post-translational regulation and allosteric regulation of pyruvate dehydrogenase and carboxylase to maintain and support the partition of pyruvate synthesis and degradation. Discovery of structure Coenzyme A was identified by Fritz Lipmann in 1946, who also later gave it its name. Its structure was determined during the early 1950s at the Lister Institute, London, together by L ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioester
In organic chemistry, thioesters are organosulfur compounds with the functional group . They are analogous to carboxylate esters () with the sulfur in the thioester playing the role of the linking oxygen in the carboxylate ester, as implied by the '' thio-'' prefix. They are the product of esterification between a carboxylic acid () and a thiol (). In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA.Matthys J. Janssen "Carboxylic Acids and Esters" in PATAI's Chemistry of Functional Groups: Carboxylic Acids and Esters, Saul Patai, Ed. John Wiley, 1969, New York: pp. 705–764. Synthesis The most typical route to thioester involves the reaction of an acid chloride with an alkali metal salt of a thiol: :RSNa + R'COCl -> R'COSR + NaCl Another common route entails the displacement of halides by the alkali metal salt of a thiocarboxylic acid. For example, thioacetate esters are commonly prepared by alkylation of potassium thioacetate: :CH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |