|
Penning–Malmberg Trap
The Penning–Malmberg trap (PM trap), named after Frans Michel Penning, Frans Penning and John H. Malmberg, John Malmberg, is an electromagnetism, electromagnetic device used to confine large numbers of charged particle, charged particles of a single sign of charge. Much interest in Penning–Malmberg (PM) traps arises from the fact that if the density of particles is large and the temperature is low, the gas will become a single-component plasma. While confinement of electrically neutral plasmas is generally difficult, single-species plasmas (an example of a non-neutral plasma) can be confined for long times in PM traps. They are the method of choice to study a variety of plasma phenomena. They are also widely used to confine antiparticles such as positrons (i.e., anti-electrons) and antiprotons for use in studies of the properties of antimatter and interactions of antiparticles with matter. Design and operation A schematic design of a PM trap is shown in Fig. 1. Charged partic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frans Michel Penning
Frans Michel Penning (12 September 1894 – 6 December 1953) was a Dutch experimental physicist. He received his PhD from the University of Leiden in 1923, and studied low pressure gas discharges at the Philips Laboratory in Eindhoven, developing new electron tubes during World War II. Many detailed observations of gas ionization were done with colleagues, finding notable results for helium and magnetic fields. He made precise measurements of Townsend discharge coefficients and cathode voltage fall. Penning made important contributions to the advancement of high resolution mass spectrometry. Biography Early life and education Penning was born in west Netherlands in the town of Gorinchem on 12 September 1894. Penning attended the University of Leiden. He studied mathematics and physics as a graduate student under Heike Kamerlingh Onnes. Penning's doctoral work involved measuring thermodynamic properties of various gases at extremely low temperatures. Penning received his PhD on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hot-filament Ionization Gauge
The hot-filament ionization gauge, sometimes called a hot-filament gauge or hot-cathode gauge, is the most widely used low-pressure (vacuum) measuring device for the region from 10−3 to 10−10 Torr. It is a triode, with the filament being the cathode. ''Note: Principles are mostly the same for hot-cathode ion sources in particle accelerators to create electrons.'' Function A regulated electron current (typically 10 mA) is emitted from a heated filament. The electrons are attracted to the helical grid by a DC potential of about +150 V. Most of the electrons pass through the grid and collide with gas molecules in the enclosed volume, causing a fraction of them to be ionized. The gas ions formed by the electron collisions are attracted to the central ion collector wire by the negative voltage on the collector (typically −30 V). Ion currents are on the order of 1 mA/ Pa. This current is amplified and displayed by a high-gain differential amplifier/electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiproton
The antiproton, , (pronounced ''p-bar'') is the antiparticle of the proton. Antiprotons are stable, but they are typically short-lived, since any collision with a proton will cause both particles to be annihilated in a burst of energy. The existence of the antiproton with electric charge of , opposite to the electric charge of of the proton, was predicted by Paul Dirac in his 1933 Nobel Prize lecture. Dirac received the Nobel Prize for his 1928 publication of his Dirac equation that predicted the existence of positive and negative solutions to Einstein's energy equation (E = mc^2) and the existence of the positron, the antimatter analog of the electron, with opposite charge and spin. The antiproton was first experimentally confirmed in 1955 at the Bevatron particle accelerator by University of California, Berkeley physicists Emilio Segrè and Owen Chamberlain, for which they were awarded the 1959 Nobel Prize in Physics. In terms of valence quarks, an antiproton consists of two ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
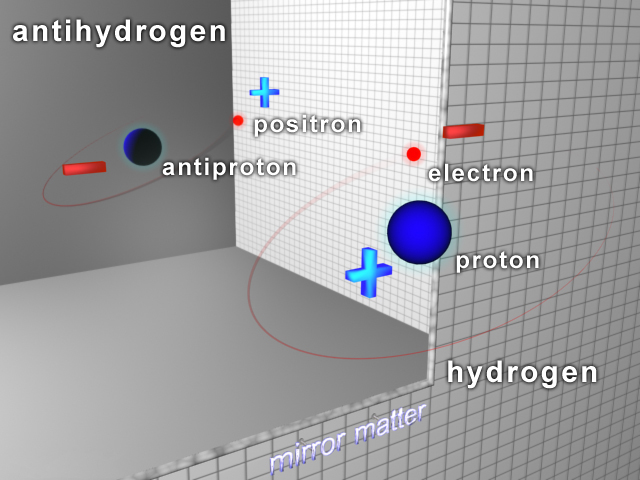
Antihydrogen
Antihydrogen () is the antimatter counterpart of hydrogen. Whereas the common hydrogen atom is composed of an electron and proton, the antihydrogen atom is made up of a positron and antiproton. Scientists hope that studying antihydrogen may shed light on the question of why there is more matter than antimatter in the observable universe, known as the baryon asymmetry problem. Antihydrogen is produced artificially in particle accelerators. Experimental history Accelerators first detected hot antihydrogen in the 1990s. ATHENA studied cold in 2002. It was first trapped by the Antihydrogen Laser Physics Apparatus (ALPHA Collaboration, ALPHA) team at CERN in 2010, who then measured the structure and other important properties. ALPHA, AEGIS, and GBAR plan to further cool and study atoms. 1s–2s transition measurement In 2016, the ALPHA experiment measured the atomic electron transition between the two lowest energy levels of antihydrogen, 1s–2s. The results, which are identical t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Positronium
Positronium (Ps) is a system consisting of an electron and its antimatter, anti-particle, a positron, bound together into an exotic atom, specifically an onium. Unlike hydrogen, the system has no protons. The system is unstable: the two particles annihilate each other to predominantly produce two or three gamma-rays, depending on the relative spin states. The energy levels of the two particles are similar to that of the hydrogen atom (which is a bound state of a proton and an electron). However, because of the reduced mass, the frequency, frequencies of the spectral lines are less than half of those for the corresponding hydrogen lines. States The mass of positronium is 1.022 MeV, which is twice the electron mass minus the binding energy of a few eV. The lowest energy orbital state of positronium is 1S, and like with hydrogen, it has a hyperfine structure arising from the relative orientations of the spins of the electron and the positron. The Singlet state, ''singlet' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Entanglement
Quantum entanglement is the phenomenon that occurs when a group of particles are generated, interact, or share spatial proximity in a way such that the quantum state of each particle of the group cannot be described independently of the state of the others, including when the particles are separated by a large distance. The topic of quantum entanglement is at the heart of the disparity between classical and quantum physics: entanglement is a primary feature of quantum mechanics not present in classical mechanics. Measurements of physical properties such as position, momentum, spin, and polarization performed on entangled particles can, in some cases, be found to be perfectly correlated. For example, if a pair of entangled particles is generated such that their total spin is known to be zero, and one particle is found to have clockwise spin on a first axis, then the spin of the other particle, measured on the same axis, is found to be anticlockwise. However, this behavior gives ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inviscid
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water. Viscosity quantifies the internal frictional force between adjacent layers of fluid that are in relative motion. For instance, when a viscous fluid is forced through a tube, it flows more quickly near the tube's axis than near its walls. Experiments show that some stress (such as a pressure difference between the two ends of the tube) is needed to sustain the flow. This is because a force is required to overcome the friction between the layers of the fluid which are in relative motion. For a tube with a constant rate of flow, the strength of the compensating force is proportional to the fluid's viscosity. In general, viscosity depends on a fluid's state, such as its temperature, pressure, and rate of deformation. However, the dependence on some of these properties is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decay Of Pure Electron Plasma
Decay may refer to: Science and technology * Bit decay, in computing * Software decay, in computing * Distance decay, in geography * Decay time (fall time), in electronics Biology * Decomposition of organic matter * Tooth decay (dental caries), in dentistry * Mitochondrial decay, in genetics Physics * Orbital decay, the process of prolonged reduction in the height of a satellite's orbit * Particle decay * Radioactive decay * Optical decay, in quantum physics Mathematics * Exponential decay Psychology and sociology * Decay theory, in psychology and memory * Social decay (decadence), in sociology * Urban decay, in sociology Entertainment * Network decay (channel drift), in television programming * Decay (DC Comics), a comic book character * '' Half-Life: Decay'', a 2001 video game add-on * Deekay, a Danish production team * Decay (professional wrestling), a professional wrestling stable in TNA Wrestling Film * ''Decay'' (2012 film), a 2012 zombie film set at the Larg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium Gas
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling and melting point are the lowest among all the elements. It is the second lightest and second most abundant element in the observable universe ( hydrogen is the lightest and most abundant). It is present at about 24% of the total elemental mass, which is more than 12 times the mass of all the heavier elements combined. Its abundance is similar to this in both the Sun and in Jupiter, due to the very high nuclear binding energy (per nucleon) of helium-4, with respect to the next three elements after helium. This helium-4 binding energy also accounts for why it is a product of both nuclear fusion and radioactive decay. The most common isotope of helium in the universe is helium-4, the vast majority of which was formed durin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |